Middle/low concentration positive quality control liquid for urine analysis
A positive quality control and urine analysis technology, applied in the field of quality control solution, can solve the problems of difficult preparation of magnesium acetoacetate, high risk of biological contamination, and limited sources of quality control substances, so as to reduce mutual interference and reduce biological risk , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Borate buffer solution with pH 5.7, 60mmol / L;
[0124] Tween-20, 40mmol / L;
[0125] Trehalose, 50mmol / L;
[0126] K30, 40mmol / L;
[0127] Glucose item: anhydrous glucose, 500mg / L;
[0128] Albumin item: bovine serum albumin, 300mg / L;
[0129] Occult blood items: bovine hemoglobin, 1mg / L;
[0130] White blood cell items: esterase, 12mg / L;
[0131] Ketone body item: acetone, 150mg / L;
[0132] Bilirubin item: 1-naphthylamine hydrochloride, 12mg / L;
[0133] Urobiliol original item: 2,5-dimethylpyrrole, 15mg / L;
[0134] Nitrite item: nitrite, 12mg / L;
[0135] Creatinine item: creatinine, 500mg / L;
[0136] Urinary calcium items: anhydrous calcium chloride, 500mg / L.
[0137] The positive quality control solution of the present invention is prepared by conventional methods. The prepared quality control solution is colorless and transparent.
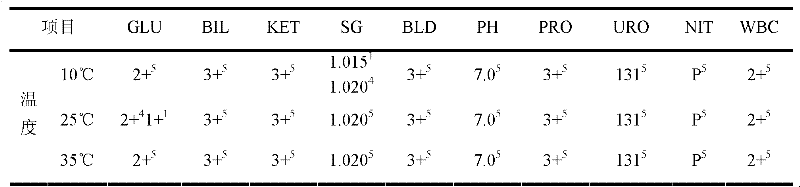
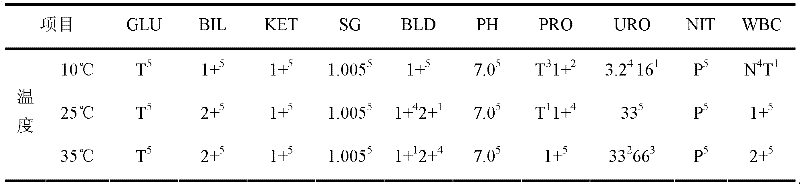
[0138] In order to illustrate the effect of the quality control solution of the present invention, a comparative experiment w...
Embodiment 2
[0148] Borate buffer solution with a pH of 7.1, 135mmol / L;
[0149] Tween-20, 100mmol / L;
[0150] Trehalose, 130mmol / L;
[0151] K30, 160mmol / L;
[0152] Anhydrous glucose, 990mg / L;
[0153] Bovine serum albumin, 800mg / L;
[0154] Bovine hemoglobin, 3mg / L;
[0155] Esterase, 18mg / L;
[0156] Ethyl acetoacetate, 150mg / L;
[0157] 1-naphthylamine hydrochloride, 20mg / L;
[0158] 2,3-Dimethylindole, 40mg / L;
[0159] Nitrite, 20mg / L;
[0160] Creatinine, 1000mg / L;
[0161] Ascorbic acid, 250mg / L;
Embodiment 3
[0164] PH is the glycine-tris buffer solution of 6.0, 60mmol / L;
[0165] Brij35, 60mmol / L;
[0166] Lactose, 80mmol / L;
[0167] Gelatin, 80mmol / L;
[0168] Anhydrous glucose, 800mg / L;
[0169] Bovine serum albumin, 600mg / L;
[0170] Bovine hemoglobin, 2mg / L;
[0171] Esterase, 15mg / L;
[0172] Sodium ethyl acetoacetate, 150mg / L;
[0173] Direct bilirubin, 40mg / L;
[0174] Pyrrole, 40mg / L;
[0175] Nitrite, 10mg / L;
[0176] Creatinine, 1000mg / L;
[0177] Anhydrous calcium chloride, 1000mg / L.
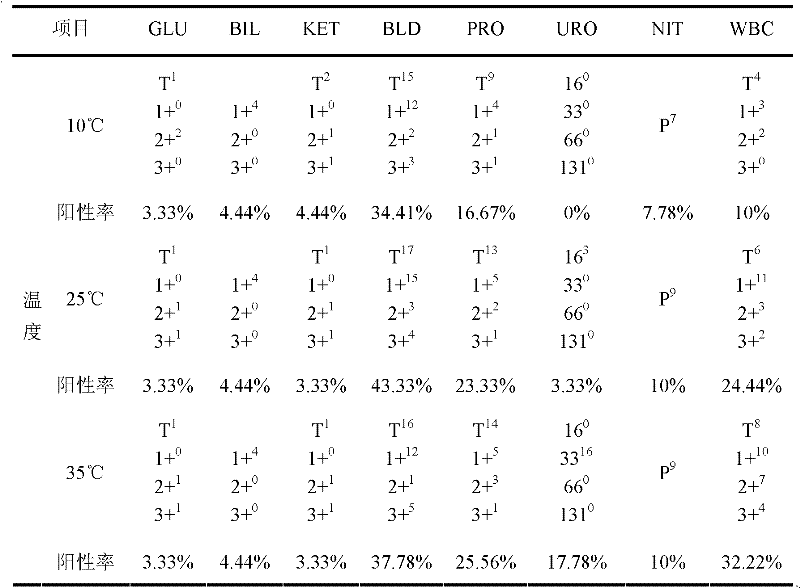
[0178] In order to illustrate the stability effect of the quality control solution of the present invention, embodiment 3 is compared with 3 comparison groups:
[0179] Comparative group 1: Briji-35, lactose, and gelatin were not added in Example 3;
[0180] Contrast group 2: do not add lactose, gelatin in embodiment 3;
[0181] Comparative group 3: No gelatin was added in Example 3.
[0182] Each comparison group was prepared at the same time as that of Example 1, and store...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com