Briarane type diterpenoid compounds with anti-tumor and antibacterial activities and application thereof
A technology for anti-tumor drugs and compounds, which can be used in anti-tumor drugs, organic active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as no anti-tumor or antibacterial activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
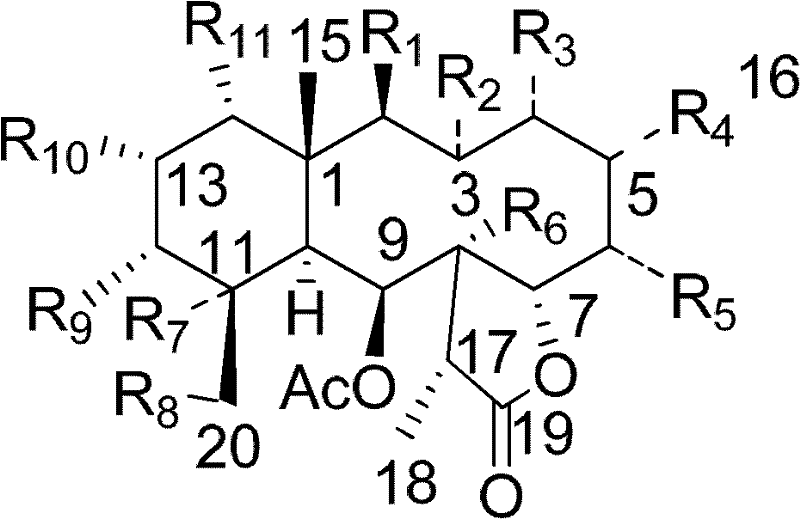
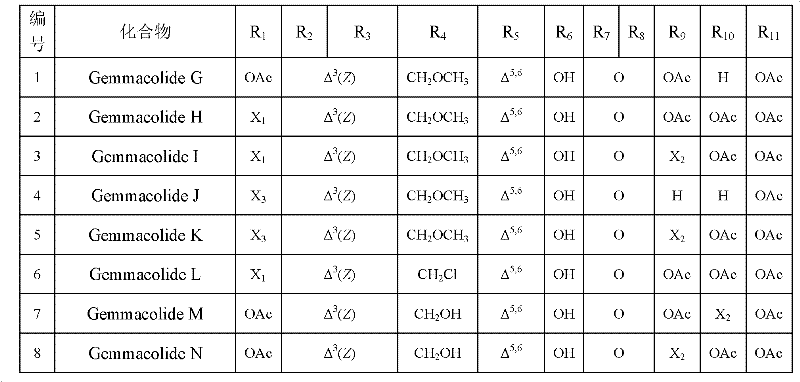
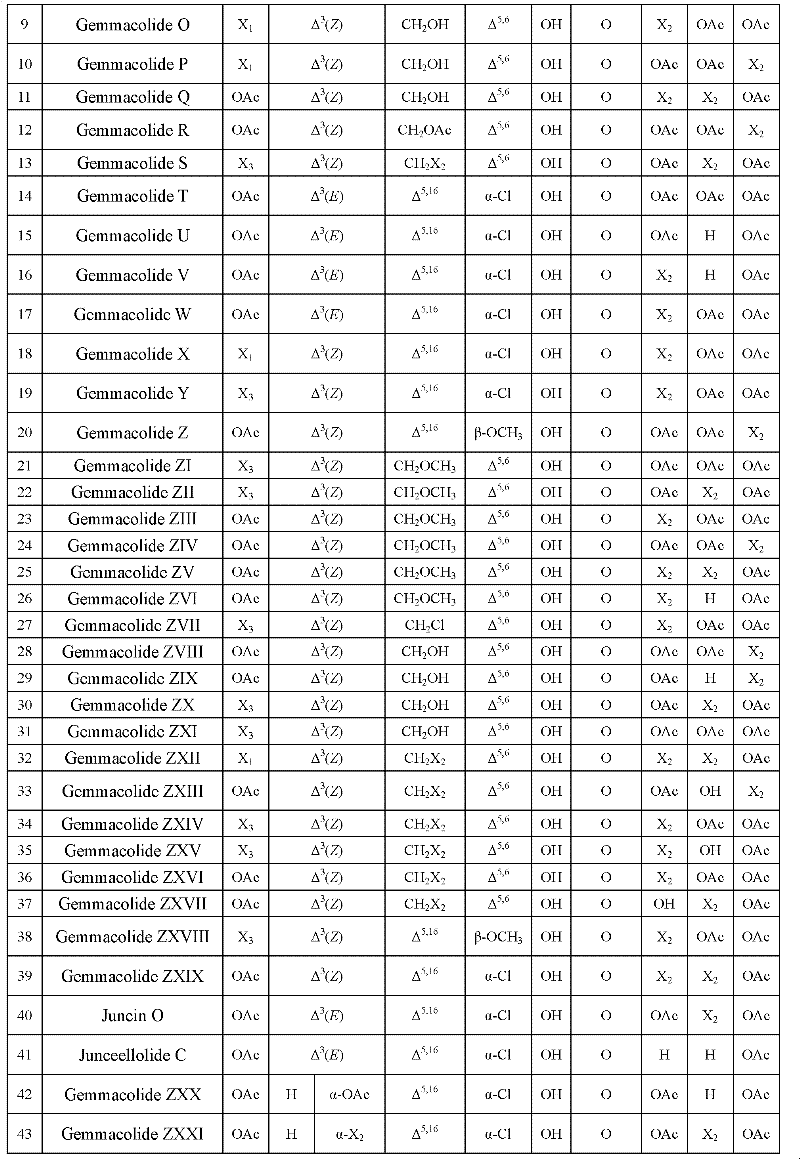
[0023] Embodiment 1. preparation compound gemmacolides G~Z, gemmacolides ZI~gemmacolide ZXXXIII, Juncin O and Junceellolide C
[0024] 1. Preparation: crush the gorgonian coral (Dichotella gemmacea) with a wet weight of 3.5Kg, and extract it with 2L acetone ultrasonically as usual, and concentrate the extract under reduced pressure to obtain a total crude extract of 29.6g, then suspend and disperse with water, and separate Extracted 5 times with ethyl acetate and n-butanol, and concentrated under reduced pressure to obtain 16.1 g of ethyl acetate extract and 3.6 g of n-butanol extract, respectively. Then the ethyl acetate extraction extract was suspended and dispersed in 1.5L methanol, extracted 4 times with n-hexane, and the n-hexane extract and the methanol layer were concentrated under reduced pressure respectively to obtain 3.7 g of n-hexane extract extract and 3.7 g of methanol layer extract. Cream 11.2g. Then 11.2g of the methanol layer extract was separated by normal p...
Embodiment 2
[0163] Example 2. Antitumor experiments of diterpenoids 1 to 55 (Gemmacolides G to Z, Juncin O, Junceellolide C, gemmacolides ZI to gemmacolide ZXXXIII) of the present invention
[0164] 1. Experimental method
[0165] The compound of the present invention was tested for inhibition of tumor cell proliferation by conventional MTT method (Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55-63).
[0166] 1. Cell lines used in the experiment: A549 (human lung cancer cells), U251 (human glioma cells) and MG63 (human osteosarcoma cells). The cell lines used in the experiments were from the Institute of Cells, Chinese Academy of Sciences.
[0167] 2. Experimental reagents, consumables and instruments:
[0168]DMEM medium (Invitrigen); 1640 medium (Invitrigen); McCoy's 5a (Invitrigen); serum (Invitrigen); trypsin (Invitrigen); DMSO (sigma); dish (Corning); pipette (Corning); 96...
Embodiment 3
[0185] Embodiment 3. the antibacterial experiment of diterpenoids 1~55 (Gemmacolides G~Z, Juncin O, Junceellolide C, gemmacolides ZI~gemmacolide ZXXXIII) of the present invention
[0186] 1. Experimental method
[0187] The compound of the present invention is carried out in vitro antibacterial test by agar diffusion method
[0188] 1. Experimental bacteria (provided by the Marine Drug Research Center of the Second Military Medical University)
[0189] Fungi: Microbotryum violaceum, Septoria tritici;
[0190] Bacteria: Escherichia coli, Bacillus megaterium.
[0191] 2. Experimental drugs
[0192] 1) Positive control drugs: penicillin (purchased from Harbin Pharmaceutical Group General Pharmaceutical Factory), streptomycin (purchased from North China Pharmaceutical Co., Ltd.), ketoconazole (purchased from North China Pharmaceutical Co., Ltd. company);
[0193] 2) Negative reference substance: acetone (Acetone) (purchased from Sinopharm Chemical Reagent Co., Ltd.);
[0194...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com