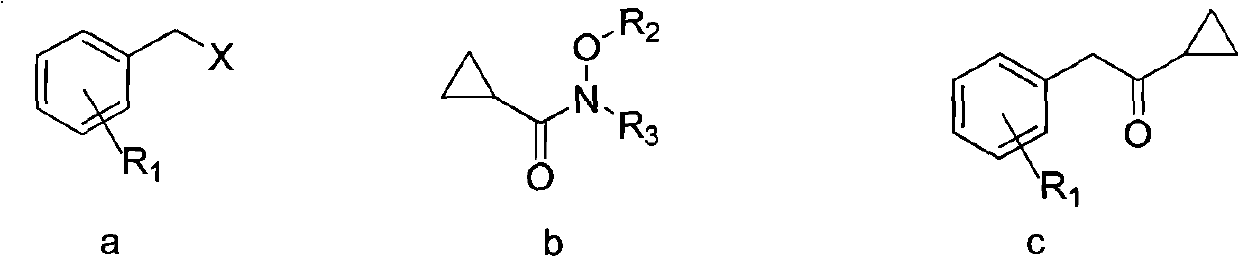
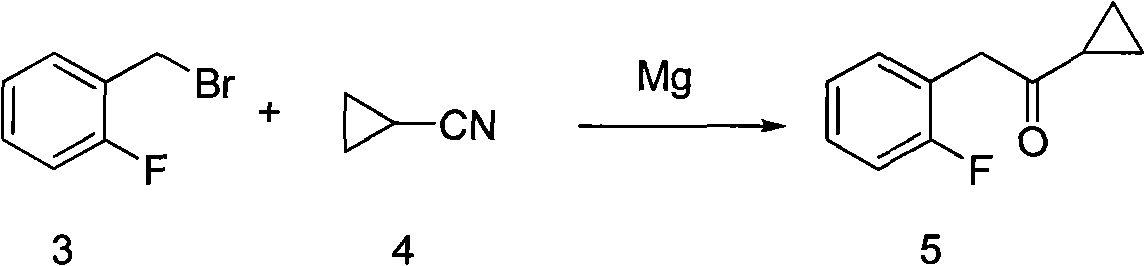
Preparation method and application of aromatic cyclopropyl butanone compound
A cyclopropyl ethyl ketone and compound technology, which is applied in the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of complex post-processing, easy to generate danger, etc., and achieve easy processing and easy reaction. The effect of progress and complete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 1-cyclopropyl-2-(2-fluorophenyl)ethanone
[0035] Weigh magnesium (2.88g, 0.12mol), put it into a three-necked reaction flask, under the protection of nitrogen, add 120mL of tetrahydrofuran, activate the magnesium with 0.1mL of 1,2-dibromoethane at 25°C, after the activation is completed, slowly add 1 -(Bromomethyl)-2-fluorobenzene (18.9g, 0.10mol), the internal temperature was controlled at 20-25°C. After the dropwise addition, continue to react at this temperature for about 2 hours, and monitor the reaction by GC until the end of the reaction. At 0° C., a solution of N-methoxy-N-methylcyclopropylcarboxamide (12.9 g, 0.10 mol) dissolved in 200 ml of tetrahydrofuran was added dropwise. After the addition was complete, the reaction was tracked by TLC until the reaction was completed. Saturated ammonium chloride was added, liquid separation, extraction, solvent was evaporated, and the obtained organic layer liquid was distilled under reduced pre...
Embodiment 2
[0037] Example 2: Preparation of 1-cyclopropyl-2-(2-fluorophenyl)ethanone
[0038]Weigh magnesium powder (2.88g, 0.12mol), put it into a three-necked reaction flask, under nitrogen protection, add 120mL of tetrahydrofuran, activate magnesium with 0.1mL 1,2-dibromoethane at 25°C, after the activation is completed, slowly add 1-(Chloromethyl)-2-fluorobenzene (14.46g, 0.10mol), the internal temperature was controlled at 20-25°C. After the dropwise addition, continue to react at this temperature for about 2 hours, and monitor the reaction by GC until the end of the reaction. At 0° C., a solution of N-methoxy-N-methylcyclopropylcarboxamide (12.9 g, 0.10 mol) dissolved in 200 ml of tetrahydrofuran was added dropwise. After the dropwise addition, the reaction was tracked by TLC until the reaction was completed. Saturated ammonium chloride was added, liquid separation, extraction, solvent was evaporated, and the obtained organic layer liquid was distilled under reduced pressure to ob...
Embodiment 3
[0039] Embodiment 3: the preparation of 2-(3-chlorophenyl)-1-cyclopropyl ethyl ketone
[0040] Operate in the same way as in Example 1, weigh 1-chloro-3-(chloromethyl)benzene (16.1g, 0.10mol), and add N-ethoxyl dissolved in 200ml tetrahydrofuran dropwise at 0°C -N-Isopropylcyclopropanecarboxamide (17.1g, 0.10mol) solution to obtain 16.6g of 2-(3-chlorophenyl)-1-cyclopropylethanone with a yield of 85.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com