Preparation method of Eslicarbazepine
A technology of chiral catalyst and hydrogen source, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalyst, organic chemistry, etc., can solve separation and purification difficulties, reduce solvent recovery utilization rate, and use a large amount of catalyst and other issues, to achieve the effect of high atom economy, atom economy, green environmental protection, and high recycling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
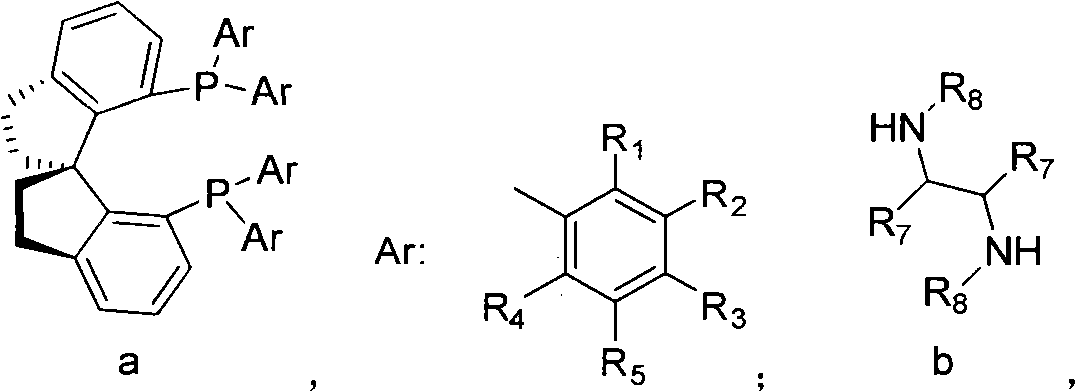
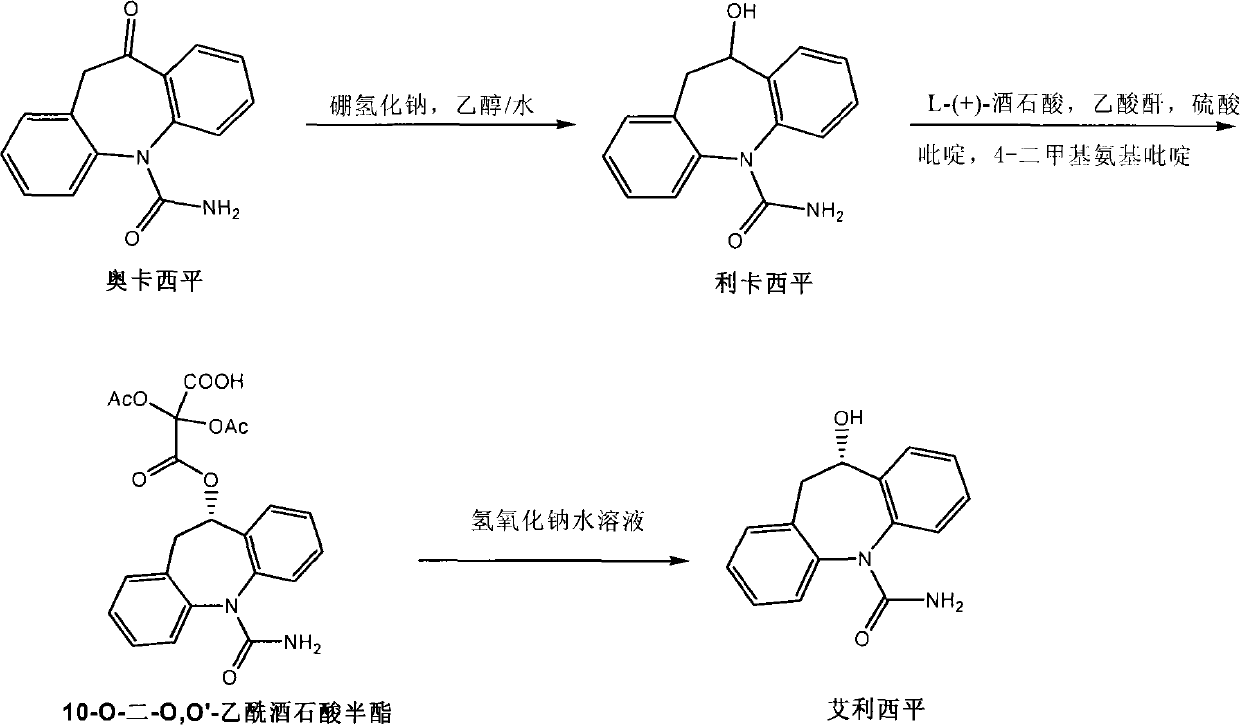
[0047] Evacuate the 1L autoclave to 2 ((S)-Xyl-SDP)((R,R)-DPEN)](RuCl 2 (p-cymene) and (R, R-DPEN) are from reagent companies, (S)-Xyl-SDP is from Zhejiang Jiuzhou Pharmaceutical Co., Ltd.) and 500ml of degassed absolute ethanol. Use high-purity nitrogen to change the gas in the kettle three times, and then replace nitrogen with high-purity hydrogen three times. Heating to 55-60°C, and then leading to high-purity hydrogen to 10.0Mpa, keeping for 48 hours. Sampling analysis showed that oxcarbazepine was <0.10%. Drain the hydrogen in the kettle, replace the hydrogen in the kettle with nitrogen three times, transfer the reaction solution to a 1000ml ordinary reaction bottle, concentrate the ethanol, stop the concentration when about 450ml is evaporated, and add 500ml of pure water while it is hot. Raise the temperature to reflux, then slowly cool to 5-10°C, and keep it for 2 hours, filter, wash the filter cake with iced ethanol water (1:10), and dry the product under vacuum at ...
Embodiment 2
[0049] Evacuate the 1L autoclave to 2 ((S)-Tol-SDP)((R,R)-DPEN)](RuCl 2 (p-cymene) and (R, R-DPEN) come from Reagent Company, (S)-Tol-SDP comes from Zhejiang Jiuzhou Pharmaceutical Co., Ltd.) and 400ml of degassed anhydrous isopropanol. things. Use high-purity nitrogen to change the gas in the kettle three times, and then replace nitrogen with high-purity hydrogen three times. Heating to 70-80°C, and then leading to high-purity hydrogen to 5.0Mpa, keeping until oxcarbazepine<0.10% to end the reaction. Drain the hydrogen in the kettle, replace the hydrogen in the kettle with nitrogen three times, transfer the reaction solution to a 1000ml common reaction bottle, concentrate the isopropanol, stop the concentration when about 350ml is evaporated, and add 500ml of pure water while it is hot. Raise the temperature to reflux, then slowly cool to 5-10°C, and keep it for 2 hours, filter, wash the filter cake with icy isopropanol water (1:10), and dry the product under vacuum at 50°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com