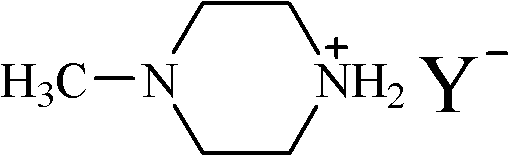
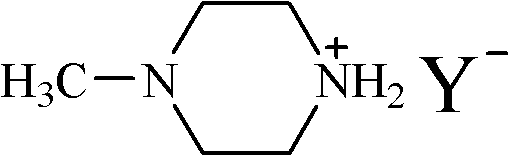
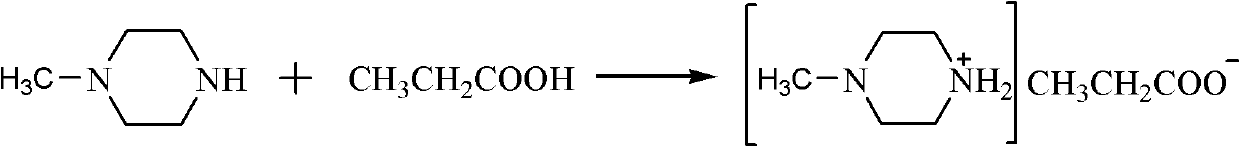Ionic liquid of N-methyl piperazine salt and preparation method thereof
A methylpiperazine and salt ion technology is applied in the field of N-methylpiperazine salt ionic liquid and its preparation, and achieves the effects of high yield, mild reaction conditions and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The propionate ionic liquid of embodiment 1N-methylpiperazine
[0018] A solution of propionic acid (7.41 g, 0.1 mol) in ethanol (50 mL) was slowly added dropwise to a solution of N-methylpiperazine (10.02 g, 0.1 mol) in ethanol (100 mL) under an ice-water bath, and reacted for 1 hour. Then the temperature was raised to 30° C., and the reaction was carried out for 3 hours. After the reaction was completed, the solvent was removed by rotary evaporation to obtain a light yellow solid. Recrystallized with ethanol / ether mixed solvent at 40°C to obtain white needle-like crystals. After vacuum drying at 40°C, 15.67 g of pure N-methylpiperazine propionate was obtained with a yield of 90% and a melting point of 71.7°C.
[0019] 1 H NMR (500MHz, CDCl 3 ): δ8.90(s, 2H), 3.04(t, 4H), 2.57(m, 4H), 2.33(s, 3H), 2.25(q, 2H), 1.11(t, 3H)
[0020] The reaction formula is:
[0021]
Embodiment 2
[0022] The lactate ionic liquid of embodiment 2N-methylpiperazine
[0023] N-methylpiperazine (10.02 g, 0.1 mol) in ethanol (30 mL) was slowly added dropwise to lactic acid (9.01 g, 0.1 mol) in ethanol (100 mL), and stirred at 40°C for 4 hours. After the reaction, the solvent was removed by rotary evaporation. Impurities and unreacted raw materials were evaporated under low pressure at 80°C to obtain lactate ionic liquid of light yellow viscous N-methylpiperazine with a yield of 85% and a melting point below -20°C.
[0024] 1 H NMR (500MHz, CDCl 3 ): δ6.02(broad, 3H), 4.05(q, H), 3.12(t, 4H), 2.63(m, 4H), 2.34(s, 3H), 1.36(d, 3H)
[0025] The reaction formula is:
[0026]
Embodiment 3
[0027] The methanesulfonate ionic liquid of embodiment 3N-methylpiperazine
[0028] The ethanol solution (30mL) of methanesulfonic acid (4.81g, 0.05mol) was slowly added dropwise to the ethanol (50mL) solution of N-methylpiperazine (5.01g, 0.05mol) under ice-water bath, and the methanesulfonic acid After the solution was added dropwise, the temperature was raised to 30° C., and the reaction was carried out for 5 hours. After the reaction, part of the solvent was removed by rotary evaporation under low pressure, and then a certain amount of diethyl ether was added, crystals were formed at 0°C, the white solid was obtained by filtration, and vacuum-dried at 30°C for 8 hours to obtain methanesulfonic acid of pure N-methylpiperazine Salt ionic liquid, the yield is 95%. The melting point is 42.1°C, 1 H NMR (500MHz, CDCl 3 ): δ3.25(s, 2H), 3.16(t, 4H), 2.79(s, 3H), 2.68(m, 4H), 2.35(s, 3H).
[0029] The reaction formula is:
[0030]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com