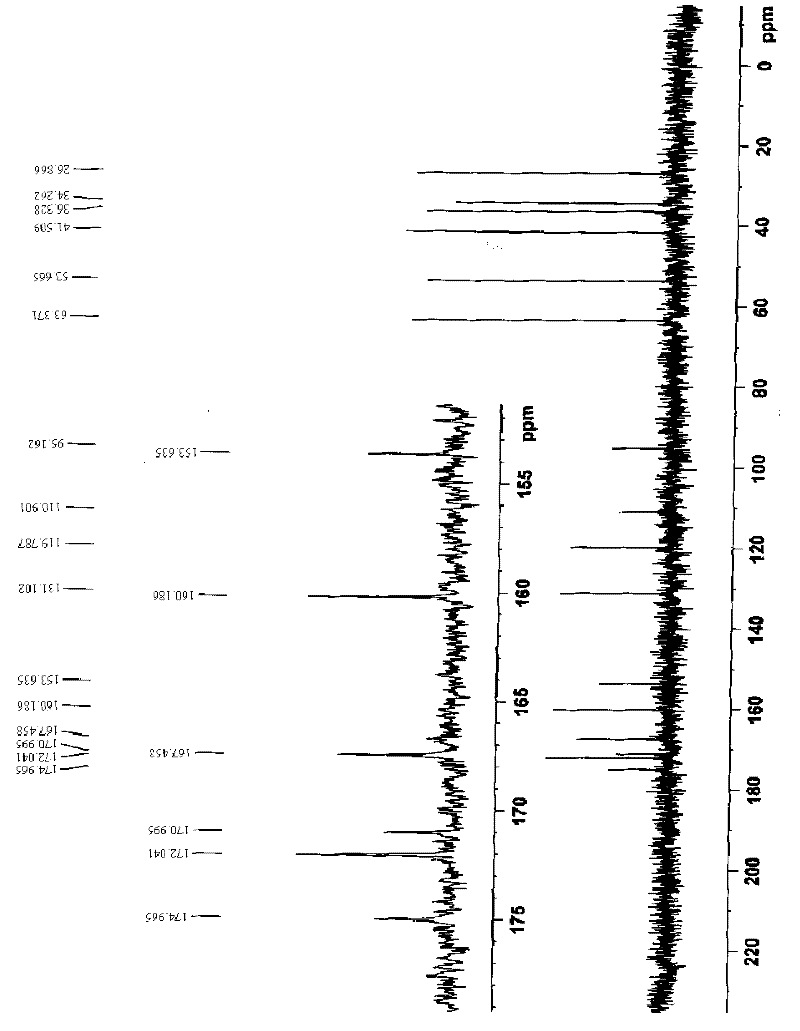
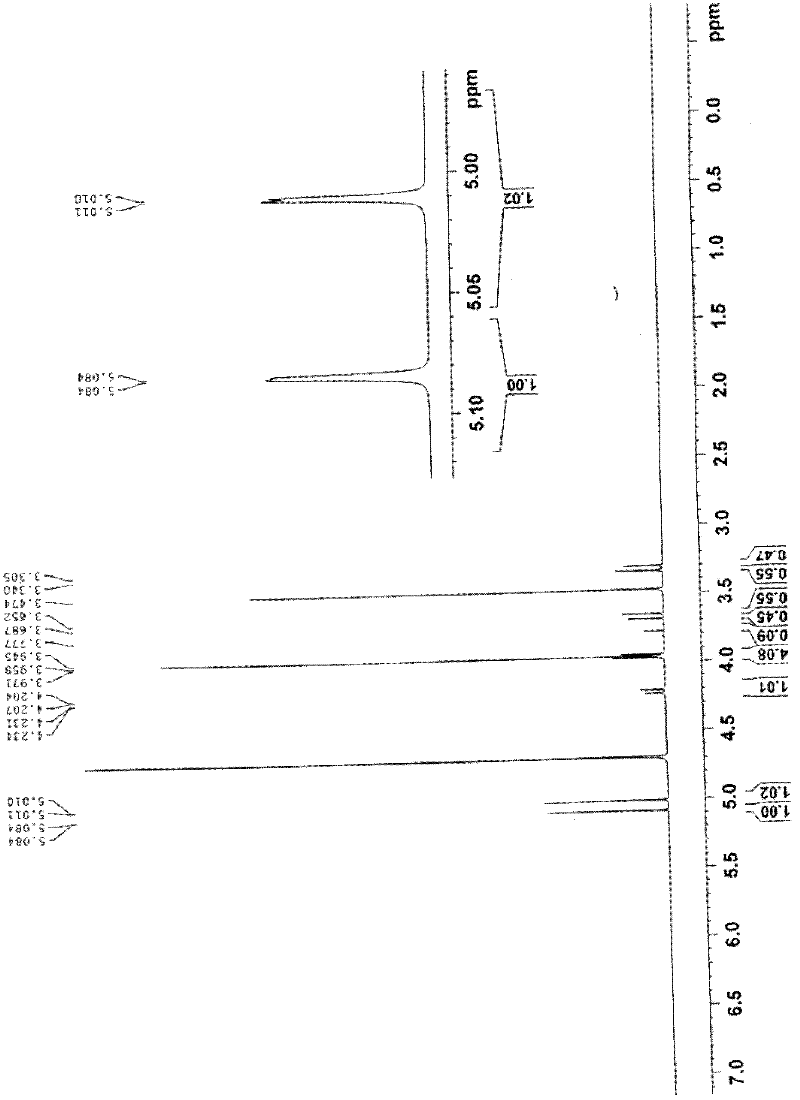
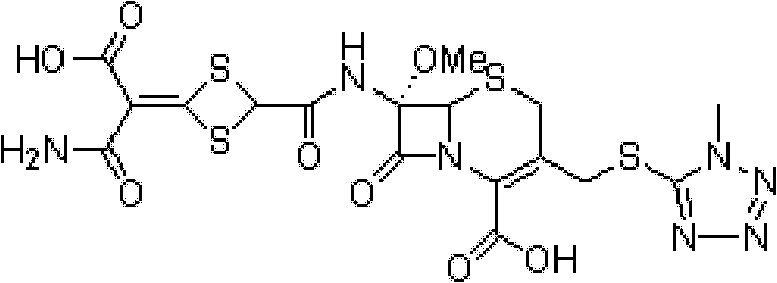
Preparation method of cefotetan
A technology of cefotetan and intermediates, applied in the direction of organic chemistry and the like, can solve the problems of high quality, low product yield and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] 1) Preparation of intermediate CefoD-1
[0090] Add the organic solvents dichloromethane and 7-MAC into the cleaned and dried glass-lined reaction tank, stir and cool to -20°C, and slowly add the alkaline reagent pyridine dropwise for about 15 minutes. Cool to -25°C, add chloroacetyl chloride dropwise, and complete the addition in about 10 to 20 minutes, and control the temperature during the dropwise addition to not exceed -15°C. Insulate and stir for 15 minutes, then take a sample for inspection (7-MAC≤2.0%, if the reaction is not complete, prolong the reaction time until the reaction is complete).
[0091] Add purified water, sodium chloride, and concentrated hydrochloric acid into a glass-lined reaction tank, and stir until completely dissolved. Transfer the solution into a glass-lined reaction tank that has been completely reacted. During the transfer process, the temperature should not exceed 5°C. layer, and the upper water phase is sent to the sewage treatment ...
Embodiment 2
[0121] 1) Preparation of intermediate CefoD-1
[0122] The process includes reaction, hydrolysis, decolorization and crystallization, in which:
[0123] The reaction described is: add 7.52kg of organic solvents dichloromethane and 1kg of 7-MAC into the reaction tank, stir and cool to -20°C, slowly add 0.2kg of alkaline reagent pyridine dropwise, after 15 minutes, cool to -25°C and then Add 0.25kg of chloroacetyl chloride dropwise, and finish adding in 10-20 minutes. During the dropping process, control the temperature not to exceed -15°C, keep warm and stir for 15 minutes, and send samples for inspection. If 7-MAC≤2.0%, the reaction is complete; if 7-MAC> 2.0%, the reaction is not complete, prolong the reaction time until the reaction is complete.
[0124] After the reaction is complete, carry out hydrolysis, decolorization and crystallization.
[0125] Described hydrolysis comprises the steps:
[0126] (i) Add purified water, sodium chloride and concentrated hydrochloric a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com