Ursolic acid derivatives with anticancer activity and preparation method thereof
An anticancer activity, ursolic acid technology, applied in antitumor drugs, drug combinations, steroids and other directions, can solve the problems that have not yet been involved in the connection between natural α-amino acids and ursolic acid, and achieve the product with significant pharmacological activity and preparation. simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
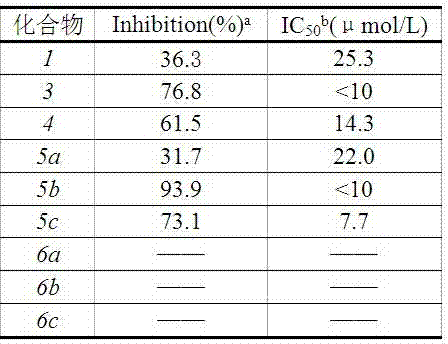
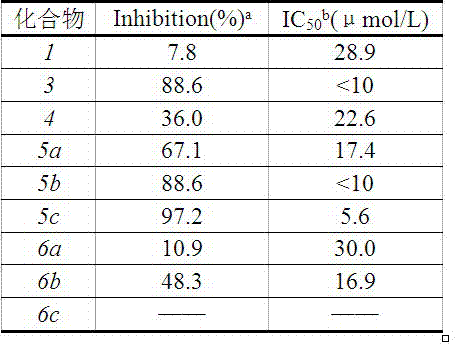
Examples
Embodiment 1
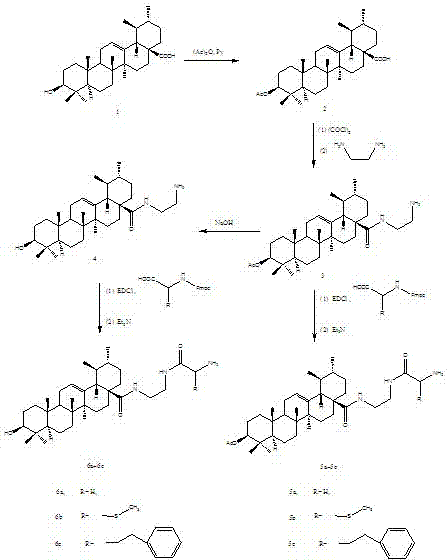
[0039] compound 2 Preparation of:
[0040]10 g of ursolic acid was dissolved in 45 mL of pyridine, and 25 mL of acetic anhydride was slowly added dropwise in an ice bath, and the reaction was carried out at room temperature for 8 h after dropping. Stop the reaction, evaporate pyridine under reduced pressure, dissolve the residue with dichloromethane, and wash the organic phase with hydrochloric acid (5%), distilled water, and saturated sodium chloride solution successively, and filter the organic phase after dehydrating with anhydrous sodium sulfate for 2 h , the filtrate was concentrated under reduced pressure at 45°C and then dried to constant weight, and the obtained crude product was 10.50 g. The crude product was recrystallized from absolute ethanol to obtain 8.59 g of white needle-like crystals. The crystallization yield was 83.3%, and the total yield was 80.5%. mp: 282~283°C.
[0041] 1 H NMR (600MHz, CDCl 3 ): 5.32 (t, 1H, J =3.6 Hz, H-12), 4.42 (t, 1H, J =5.6 ...
Embodiment 2
[0076] The concrete preparation method of this ursolic acid derivative is:
[0077] a: Dissolve 5 g of ursolic acid in 40 mL of pyridine, slowly add 20 mL of acetic anhydride dropwise under ice bath conditions, and react at room temperature for 6 h after the addition; stop the reaction, evaporate pyridine under reduced pressure, and wash the residue with dichloromethane Dissolve, and wash the organic phase with 5% hydrochloric acid, distilled water, and saturated sodium chloride solution successively, and then filter the organic phase with anhydrous sodium sulfate for 1 h, then concentrate the filtrate under reduced pressure at 40 °C and dry it to Constant weight, drying temperature is 60°C, compound 2 is obtained;
[0078] b: 4 g of compound 2 was completely dissolved in 30 mL of dichloromethane, and 5 mL of oxalyl chloride was slowly added dropwise under ice-bath conditions. After the dropwise addition, continued to stir in the ice bath for 0.5 h, and then continued to react...
Embodiment 3
[0085] The concrete preparation method of this ursolic acid derivative is:
[0086] a: Dissolve 15 g of ursolic acid in 50 mL of pyridine, slowly add 30 mL of acetic anhydride dropwise under ice bath conditions, and react at room temperature for 9 h after the addition; stop the reaction, evaporate pyridine under reduced pressure, and wash the residue with dichloromethane dissolved, and the organic phase was washed with 5% hydrochloric acid, distilled water, and saturated sodium chloride solution in sequence, and the organic phase was dehydrated with anhydrous sodium sulfate for 3 h, then filtered, and the filtrate was concentrated under reduced pressure at 50°C and dried to Constant weight, drying temperature is 70 ° C, to obtain compound 2;
[0087] b: 6 g of compound 2 was completely dissolved in 50 mL of dichloromethane, and 7 mL of oxalyl chloride was slowly added dropwise under ice bath conditions. After the dropwise addition, continued to stir in the ice bath for 2 h, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com