A kind of atactic/stereoblock styrenic polymer and preparation method thereof
A styrenic, multi-block technology, applied in the field of styrenic polymers, can solve the problems of difficult adjustment of tacticity, high product brittleness, high tacticity, etc. Processability, effect of good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under nitrogen protection, add 0.72mmol triisobutylaluminum and 2mmol tert-butyl chloroacetate to a 50mL catalyst preparation bottle and mix well, then add 0.04mmol 2-ethylhexanoic acid neodymium and mix well and age at 40°C for 3h to form a shallow Blue homogeneous transparent solution.
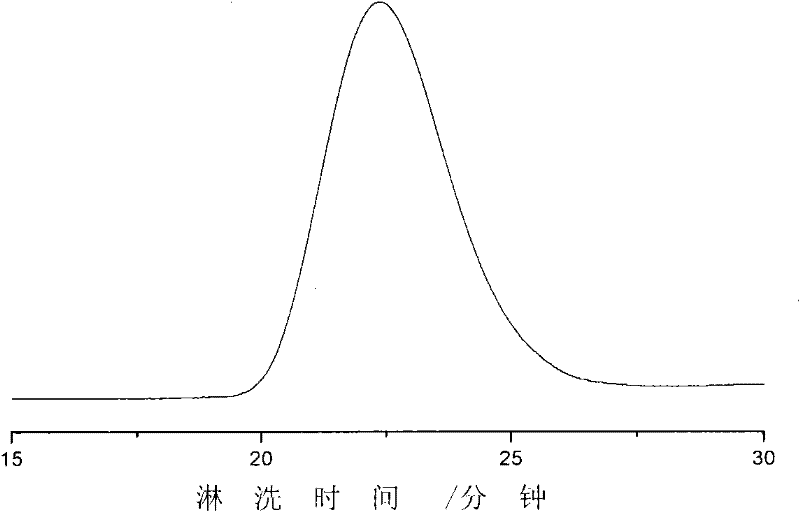
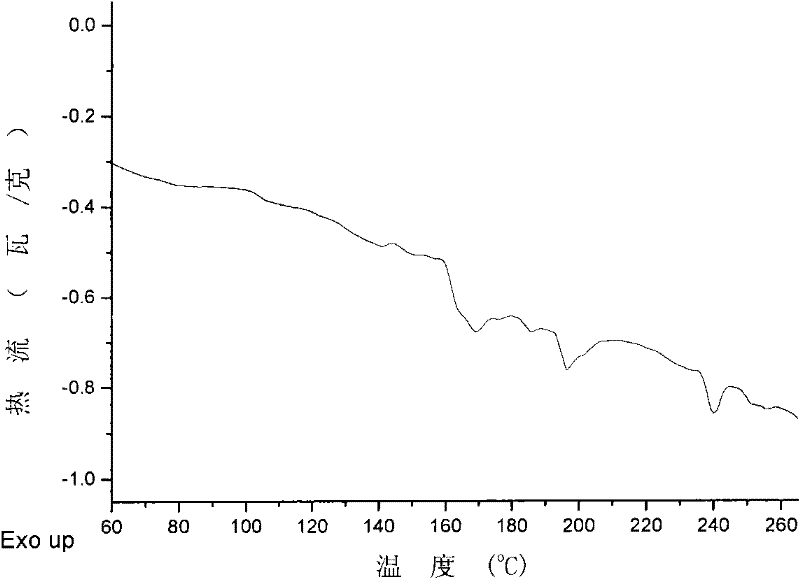
[0033] Adding styrene massfraction is 20ml of the cyclohexane solution of styrene of 26% to the polymerization bottle of 100ml drying under nitrogen protection, then adds above-mentioned catalyst solution (catalyst component A / monomer=1.0 * 10 -3 mol / mol), reacted at 40°C for 140h, added ethanol solution containing 5% hydrochloric acid by mass to terminate the reaction, washed with ethanol and dried in a vacuum oven at 50°C to constant weight to obtain 0.4g of polymer, GPC spectrum Figure presents unimodal molecular weight distribution, weight average molecular weight (M w )=1.1×10 6 g / mol, molecular weight distribution index (M w / M n ) = 1.7. The polymer sample has obvious crys...
Embodiment 2
[0035] Under nitrogen protection, add 0.75mmol triisobutylaluminum and 0.225mmol tert-butyl chloroacetate to a 50mL catalyst preparation bottle, mix them, then add 0.075mmol 2-ethylhexanoic acid neodymium and mix them, and age them at 40°C for 2h to form Light blue homogeneous transparent solution.
[0036] Styrene solution preparation mode is the same as embodiment 1, regulates styrene mass fraction to be 61%, in 20ml styrene solution, adds above-mentioned catalyst (catalyst component A / monomer=1.0 * 10 -3 mol / mol), react 72h under 60 ℃, all the other conditions are with embodiment 1, obtain polymer 0.8g, GPC spectrogram presents monomodal molecular weight distribution, weight average molecular weight (M w )=1.6×10 6 g / mol, molecular weight distribution index (M w / M n ) = 2.5. The polymer sample has obvious crystallization phenomenon, and there is a crystalline melting peak at 230 °C.
Embodiment 3
[0038] Before adding the catalyst to the monomer, add 3.75 mmol of trichloromethane as additive D to the styrene solution in the polymerization bottle under nitrogen protection and mix well. Catalyst preparation mode and all the other reaction conditions are with embodiment 2, obtain polymer 0.9g, GPC spectrogram presents monomodal molecular weight distribution, weight average molecular weight (M w )=1.1×10 6 g / mol, molecular weight distribution index (M w / M n ) = 2.8. The polymer sample has obvious crystallization phenomenon, and there are crystal melting peaks at 190 and 240 °C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Molecular weight distribution index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com