Preparation and application method of PEG recombinant pig-human urate oxidase fusion protein
A urate oxidase and urate oxidase subunit technology, which is applied in the field of preparation of PEGylated recombinant pig-human urate oxidase fusion protein, can solve problems such as allergic reactions, affecting therapeutic effects, and difficulty in maintaining drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the construction of the cloning vector that contains recombinant porcine-human urate oxidase nucleic acid molecule
[0037] Scheme 1 Whole Gene Synthesis Scheme
[0038] Carry out the whole gene synthesis of pig-human urate oxidase chimeric gene according to the following sequence:
[0039] The basic composition of the sequence is that the gene sequence of N-terminal 1-222 amino acid residues is the gene sequence of porcine urate oxidase. The gene sequence of N-terminal 2-6 amino acids is deleted, and ACC is added to the 5' end of the gene sequence to make it possible to cut the gene fragment with restriction endonuclease Nco I and reclaim it. The coding gene sequence of the 223-305 amino acid residues of the sequence is the human uric acid oxidase gene sequence, and the sequence ctc gag of restriction endonuclease Xho I is added at the end, so as to facilitate gene manipulation.
[0040] The nucleic acid sequence is:
[0041] 1 ACC ATG GAC TAC AAA AAG ...
Embodiment 2
[0115] Embodiment 2, the construction of the expression vector that contains recombinant porcine-human urate oxidase nucleic acid molecule
[0116] The expression vector plasmid pET28a was selected, and the expression host cell was Escherichia coli BL21. The build process is as follows:
[0117] 1) Double digestion of pET28a and pMD PHUO (pig-human urate oxidase), electrophoresis of the digested products, and recovery of fragments:
[0118] Nco I / Xho I double-digested pET28a, Nco I / Xho I double-digested pMD PHUO, and the double-digested products were subjected to gel electrophoresis. The pET28a fragment with Nco I / Xho I cut point and the recombinant urate oxidase chimeric gene fragment with Nco I / Xho I cut point were recovered.
[0119] 2) The connection of the pET28a fragment with the Nco I / Xho I cutting point and the recombinant urate oxidase chimeric gene fragment.
[0120] 3) Transformation of the ligation product: Escherichia coli BL21 was transformed with the ligation...
Embodiment 3
[0122] Example 3, Induced expression of recombinant pig-human urate oxidase protein
[0123] 1. Small scale culture in shake flask
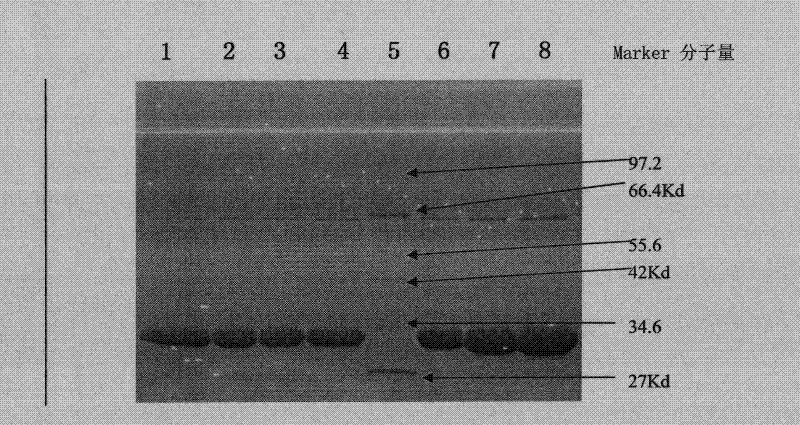
[0124] A single colony of Escherichia coli BL21 / pET28a-PHUO was picked, inoculated in 10ml of LB+Kan culture medium, and cultivated overnight. Take 1ml of the saturated bacterial solution and put it in 250ml LB+Kan culture medium, shake and culture at 37°C for 2.5h, until OD 600 =1.4~1.5, add appropriate amount of inducer α-lactose. Cultivate at 37°C for another 5 hours, and collect the bacteria by centrifugation. The addition amount of the inducer lactose was optimized several times, and the concentration of lactose was determined to be 1-10 mmol / L during shaking table fermentation, preferably 5-6 mmol / L. About 5 grams of wet bacteria can be obtained from 1L of culture solution, and 400U of crude enzyme can be obtained per gram of wet bacteria. The results of protein electrophoresis showed that the target protein accounted for more than 30% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com