A kind of chicken defensin 9 gene expression vector and its preparation method and application
A chicken defensin and gene expression technology, applied in the field of genetic engineering, can solve the problems of high cost of chemically synthesized antimicrobial peptides and lack of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Cloning of chicken defensin 9 gene
[0043] Use the primer sequence SEQ ID NO:1~4 to carry out PCR according to the following procedure respectively: each primer (20μmol / L) 1μL dNTPs (10mmol / L) 1μL, 10×buffer (Mg 2+ ) 5 μL, rTaq DNA polymerase (2.5U / μL) 1 μL, ddH 2 O was added to 50 μL. The obtained PCR product was used as a template, and the second round of PCR amplification was carried out using SEQ ID NO: 1 and SEQ ID NO: 5 as primers. The composition of the PCR reaction system is as follows: 10×buffer 5 μL, dNTPs 2 μL, primers (20 μmol / L) 2 μL each, rTaq DNA polymerase (2.5U / μL) 0.5 μL, template 1 μL, ddH 2 O to make up to 50 μL. PCR reaction program: 94°C pre-denaturation for 2min, 1 cycle; 94°C for 30s, 52°C for 30s, 72°C for 40s, 30 cycles; 72°C extension for 5min.
[0044] Specific bands at 100bp~250bp can be observed when PCR products are detected by 1.5% agarose gel electrophoresis, see Figure 4 .
Embodiment 2
[0045] Embodiment 2 Amplification of ov sequence
[0046] The total DNA of chicken oviduct was used as a template, and the ov sequence fragment was amplified with specific primers SEQ ID NO: 6-7. The composition of the PCR reaction system is as follows: 10×buffer 2.5 μL, dNTPs 2 μL, primers (20 μmol / L) 1 μL each, rTaq DNA polymerase (2.5U / μL) 0.5 μL, template 0.5 μL, ddH 2 O to 25 μL. PCR reaction program: 94°C pre-change for 5min, 1 cycle; 94°C for 40s, 55°C for 40s, 72°C for 80s, 30 cycles; 72°C extension for 10min. Using the above product as a template, PCR was performed again, and the addition amount of each item in the system was doubled, and the total volume was 50 μL.
[0047] When the PCR product is detected by 1.5% agarose gel electrophoresis, a specific band of about 1000bp can be observed, see Figure 5 .
Embodiment 3
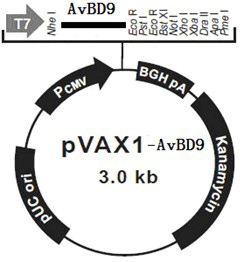
[0048] Example 3 Construction of recombinant eukaryotic expression vector pVAX1-AvBD9
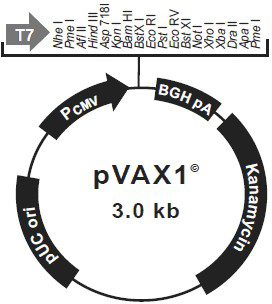
[0049] Restriction enzymes for AvBD9 gene Nhe I and EcoR I carried out enzyme digestion and digestion, and acted at 37°C for 3 hours. The reaction system was as follows: 10×buffer M 5 μL, Nhe I 1 μL, EcoR I 1 μL, PCR product 25 μL, ddH 2 O was added to 50 μL, and the pVAX1 plasmid (Invitrogen Company) was also treated with endonuclease Nhe I and EcoR I performed enzyme digestion. 37°C for 3 hours, the reaction system is as follows: 10×buffer M 5μL, Nhe I 1 μL, EcoR I 1 μL, PCR product 20 μL, ddH 2 O to 50 μL while dephosphorylated with CIAP (alkaline phosphatase). All the digested reaction solutions were electrophoresed with 1.5% agarose, and the target fragment bands were excised under ultraviolet light, and the digested target DNA fragments were recovered with a gel recovery kit. Under the action of T4 ligase, ligate the digested PCR product of AvBD9 gene and dig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com