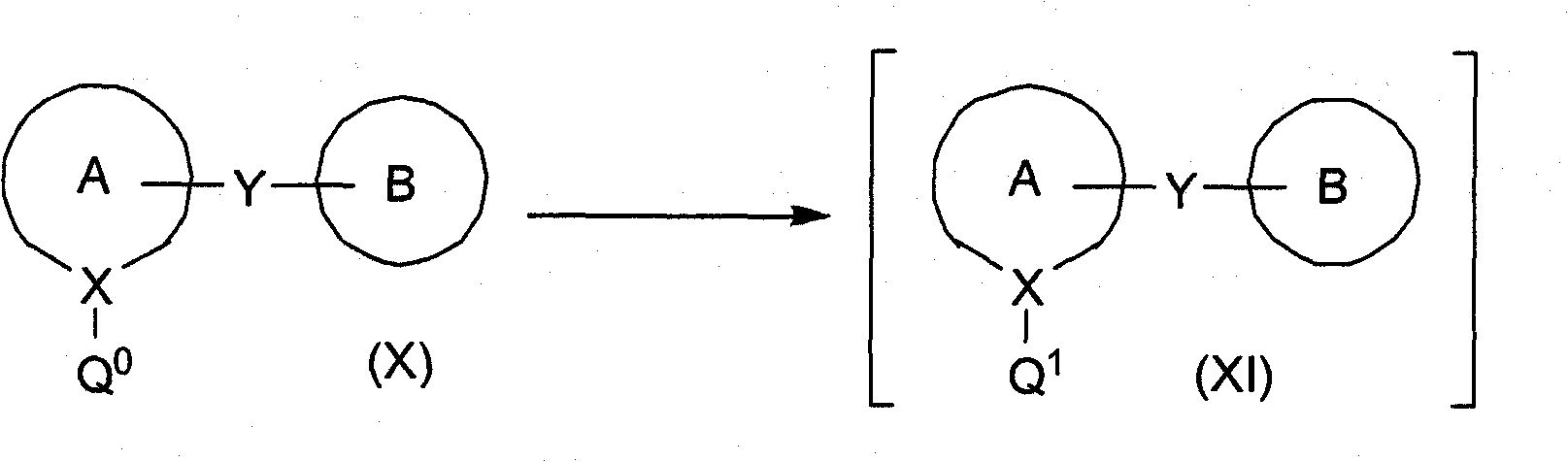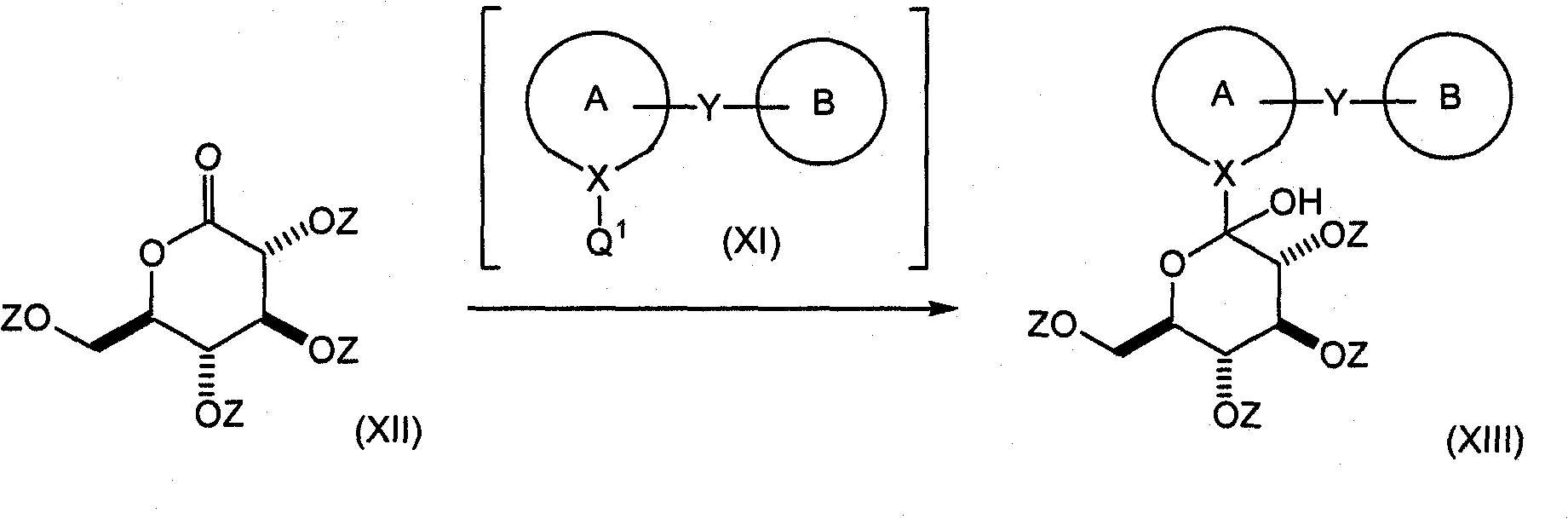Process for preparing compounds useful as sglt inhibitors
A compound and solvate technology, applied in the field of preparing compounds that can be used as SGLT inhibitors, can solve problems such as destroying and deteriorating cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
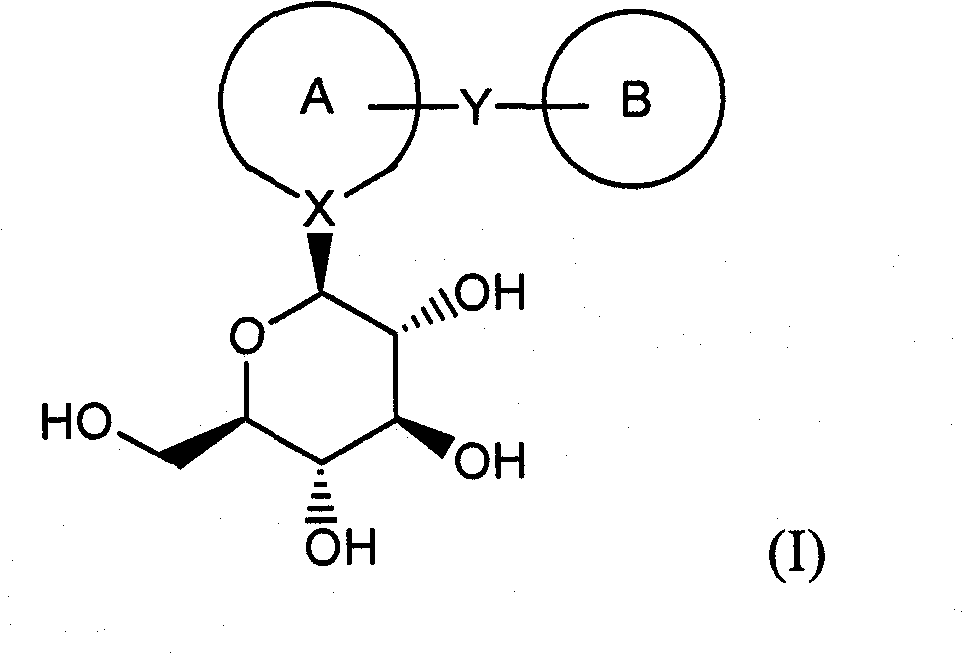
[0272] The present invention relates to a process for the preparation of compounds of formula (I), as shown in Scheme 1 below.
[0273]
[0274] Correspondingly, let suitably substituted formula (X) compound (wherein Q 0 is bromine or iodine; which are known compounds or compounds prepared by known methods) and bis(C 1-4 Alkyl) magnesium-lithium chloride complexes (such as di(sec-butyl) magnesium-lithium chloride, etc.) or C 1-4 Alkylmagnesium chloride-lithium chloride complex or C 1-4 Alkyl magnesium bromide-lithium chloride complex (wherein C 1-4 The alkyl group is preferably isopropyl or sec-butyl, more preferably sec-butyl; it is a known compound or a compound prepared by a known method) reaction; wherein two (C1-4 Alkyl) magnesium-lithium chloride complex or C 1-4 Alkylmagnesium chloride-lithium chloride complex or C 1-4 The alkylmagnesium bromide-lithium chloride complex is preferably present in the range of about 1.0 to 1.5 molar equivalents (relative to the mole...
example 1
[0422] Example 1: Acetic acid-3(R), 4(S), 5(R)-triacetoxy-6-{3-[5-(4-fluorophenyl)-thiophene -2-ylmethyl]-4-methyl-phenyl}-6-hydroxy-tetrahydropyran-2(R)-ylmethyl ester
[0423]
[0424] Step A: Preparation of Grignard Reagent
[0425] 2-(4-Fluorophenyl)-5-(5-iodo-2-methyl-benzyl)thiophene (122.48 g, 0.3 mol) was stirred in toluene (0.75 L / mol) at ambient temperature, then Cool to -10°C. Then, sec-butylmagnesium chloride lithium chloride (about 15% solution in THF; 269.70 g, 0.36 mol ), and the resulting dark green solution was stirred between -5 °C and 0 °C for 1 h.
[0426] Step B :
[0427]Dilute acetate-3(R),4(S),5(R)-triacetoxy-6-oxo-tetrahydropyran-2(R)-ylmethyl ester with THF (0.25 L / mol) ( about 50% solution in toluene, 0.39 mol), and the resulting mixture was cooled to -35°C. To this mixture was then added the solution prepared in Step A above via a syringe / addition funnel at less than about -35°C for about 1 hour under an argon atmosphere. After stir...
example 2
[0428] Example 2: Acetic acid-3(R), 4(R), 5(S)-triacetoxy-6(S)-{3-[5-(4-fluorophenyl)- Thiophen-2-ylmethyl]-4-methyl-phenyl}-tetrahydropyran-2(R)-ylmethyl ester
[0429]
[0430] Triethylsilane (87.2 g, 0.75 mol) was added to the Base)-thiophen-2-ylmethyl]-4-methyl-phenyl}-6-hydroxy-tetrahydropyran-2(R)-ylmethyl ester in acetonitrile (as prepared in Example 1 above, 0.30 mol), the resulting brown solution was cooled to 2 °C. Then, boron trifluoride etherate (46.84 g, 0.33 mol) was added via syringe over about 30 minutes, and the resulting mixture was stirred in an ice-water bath for 1 hour. To the resulting mixture was then added 10% w / w Na 2 CO 3 Aqueous solution (330ml). The resulting mixture was then heated at about 45°C until complete dissolution was observed. The layers of the resulting three-layer mixture were separated and the intermediate organic layer was allowed to cool to ambient temperature with stirring for 16 hours, during which time crystallization w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com