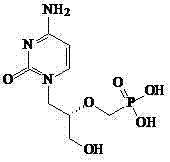
A kind of synthetic method of antiviral drug cidofovir
The technology of an antiviral drug and a synthetic method is applied in the field of preparation of the anti-cytomegalovirus drug cidofovir and its intermediate, which can solve problems such as many side reactions, unsuitability for industrial production, unsuitable purification of products, etc., and achieve simplified yield. The effect of low yield, increased yield, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
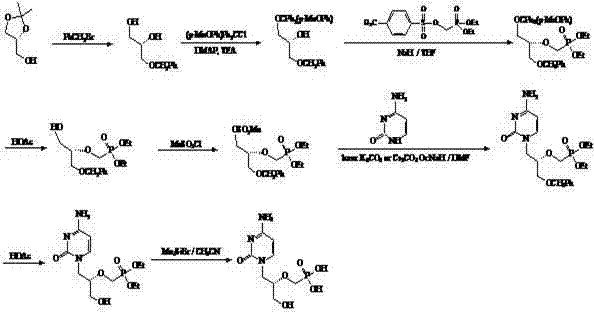
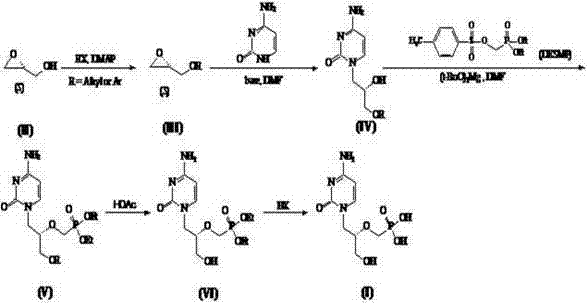
[0056] 1. Synthesis of 4,4'-dimethoxytriphenylmethyl-(R)-glycidol (compound III):
[0057] Dissolve 5.04 g (15 mmol) DMT-Cl and 0.20 g (1.52 mmol) 4-dimethylaminopyridine (DMAP) in 100 mL CH2Cl2, cool to 0 °C, add dropwise 10 mL TEA , Slowly added 2. 00 g ( 27 mmol) chiral hydroxymethyloxirane (compound II). After the addition, the reaction was naturally raised to room temperature. After 4-6 h, stop the reaction until the DMT-Cl disappears as detected by TLC. Filter and wash the filtrate with saturated NaHCO3 solution (50mL×2), saturated NaCl solution (50 mL×2), dry over anhydrous Na2SO4, filter and concentrate. A colorless viscous product, namely 5.08 g of 4,4'-dimethoxytriphenylmethyl-(R)-glycidol (compound III), was obtained directly, with a yield of 90% and a purity of 99% by HPLC.
[0058] 2. Synthesis of (S)-N1-[(2-hydroxy-3-(dimethoxytriphenylmethoxy)propyl]cytosine (compound IV):
[0059] Under nitrogen protection, 3.56 g (32 mmol) cytosine was added to 150 mL of a...
Embodiment 2
[0068] 1. Synthesis of triphenylmethyl-(R)-glycidol (compound III):
[0069] Dissolve 4.18 g (15 mmol) Tr-Cl and 0.20 g (1.52 mmol) 4-dimethylaminopyridine (DMAP) in CH2Cl2 (100 mL), cool to 0 °C, add dropwise TEA (10 mL), slowly added 2. 00 g (27 mmol) chiral hydroxymethyloxirane (compound II). After the addition, the reaction was naturally raised to room temperature. After 4-6 h, stop the reaction until the DMT-Cl disappears as detected by TLC. Filter and wash the filtrate with saturated NaHCO3 solution (50mL×2), saturated NaCl solution (50 mL×2), dry over anhydrous Na2SO4, filter and concentrate. A colorless viscous product, namely 4.22 g of triphenylmethyl-(R)-glycidol (compound III), was obtained directly, with a yield of 89% and a purity of 98% by HPLC.
[0070] 2. Synthesis of (S)-N1-[(2-hydroxy-3-(triphenylmethoxy)propyl]cytosine (compound IV):
[0071] Under nitrogen protection, 3.56 g (32 mmol) of cytosine was added to 150 mL of anhydrous N,N-dimethylformamide (D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com