The preparation method of 2-substituted-2-adamantanol compounds
An adamantanol and compound technology, applied in the field of 2-adamantanol, can solve the problems of low yield of synthesizing alkyl lithium, unsuitable for industrial production, low stability of alkyl lithium, etc., and achieves good product yield and cost Low, mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
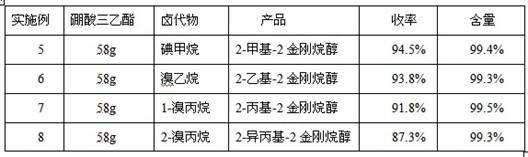
[0021] Add 30 g (0.2 mol) of 2-adamantanone, 42 g (0.4 mol) of trimethyl borate, 100 ml of n-butyl ether, and 16 g (0.24 mol) of zinc powder into a 500 ml four-necked flask. Add 34 g (0.24 mol) of iodomethane. After the addition, the temperature was raised to 60°C for 4 hours, and the reaction solution was taken for gas chromatography (GC) analysis. The content of 2-adamantanone in the reaction solution was 0.3%. What is explained here is that in industrial production, the reaction time is affected by temperature, material type, etc. and cannot be refined specifically, but in batch reaction, the reaction time is usually between 3-6 hours.
[0022] Cool the reaction solution to room temperature, add 100 ml of saturated ammonium chloride aqueous solution, stir for 0.5 hours, and then let stand to separate layers. The aqueous layer is extracted three times with n-butyl ether, and the amount of n-butyl ether for each extraction is 50 ml. After three extractions, combine The organi...
Embodiment 2
[0024] Except that 26 g (0.24 mol) of bromoethane was used instead of methyl iodide, the rest of the operation was the same as in Example 1. As a result, 33.9 g of 2-ethyl-2 adamantanol was obtained with a yield of 94.2% and a GC analysis content of 99.2%.
Embodiment 3
[0026] Except that 30 g (0.24 mol) of 1-bromopropane was used instead of methyl iodide, the rest of the operation was the same as that of Example 1. As a result, 36.3 g of 2-propyl-2-adamantanol was obtained with a yield of 93.5% and a content of 99.4% by GC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com