Phalin c and its derivatives and their application in the preparation of anticancer drugs
A derivative, kalinin technology, applied in the field of kalinin C derivatives and its preparation of anticancer drugs, can solve the problems of low biological activity and large dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
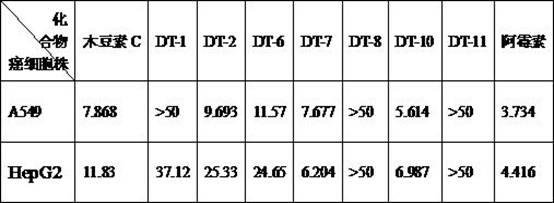
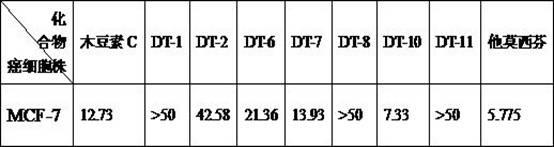
Image
Examples
Embodiment 1
[0013] Example 1. Preparation of 2-isoamyl-3,5-dimethoxy-stilbene (DT-1).
[0014] Weigh 0.5g (1.7mmol) of lignanin C, add K after grinding 2 CO 3 0.23g (1.7mmol), add 50ml of acetone, add Me dropwise under stirring 2 SO 4 0.2ml (1.7mmol), reacted at 30°C for 6h, filtered, concentrated, and purified by 200~300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 19:1 as eluent) to obtain a white solid (DT-1 ) 0.48g, the yield was 91.6%. m.p.60-61℃; 1 HNMR (CDCl 3 )δ (ppm): 1.67 (s, 3H), 1.80 (s, 3H), 3.43 (d, J=6.6Hz, 2H), 3.78 (s, 3H), 3.82 (s, 3H), 5.13 (t, J=6.6Hz,1H), 6.41(s,1H), 6.74(s,1H), 6.96(d,J=16.2Hz,1H), 7.24(t,J=7.8Hz,1H), 7.34(t, J=7.8Hz, 2H), 7.36(d, J=16.2Hz, 1H), 7.48(d, J=7.8Hz, 2H). ESI-MS m / z 309 ([M+1] + ,100%). Anal. Calcd for C 21 h 24 o 2 : C, 81.78; H,, 7.84. Found: C, 65.77; H, 7.86.
Embodiment 2
[0015] Example 2. Preparation of 2-isoamyl-3-methoxy-5-chloropropoxy-stilbene (DT-2).
[0016] Weigh 0.25g (0.85mmol) of lignanin C, add anhydrous K after grinding 2 CO 3 0.1g (0.85mmol), add DMF10ml, under the protection of nitrogen, add 0.084ml (5mmol) of 1,3-bromochloropropane dropwise, stir at 30°C for 6h, filter, add 25ml ethyl acetate to the filtrate, and then use 3×10ml water Extraction, anhydrous Na 2 SO 4 Dry overnight, filter, concentrate, and purify by 200-300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 19:1 as eluent) to obtain 0.28 g of white solid (DT-2), with a yield of 88.8% . m.p.62-63℃; 1 H NMR (CDCl 3 )δ(ppm): 1.67(s,3H), 1.80(s,3H), 2.25(m,2H), 3.43(d,J=7.2Hz,2H), 3.76(t,J=6.3Hz,2H) , 3.81(s,3H), 4.16(t,J=5.7Hz,2H), 5.12(t,J=7.2Hz,1H), 6.42(s,1H), 6.76(s,1H), 6.96(d, J=16.2Hz,1H), 7.25(t,J=7.8Hz,1H), 7.35(t,J=7.8Hz,2H), 7.36(d, J=16.2Hz,1H), 7.50(d,J= 7.8Hz, 2H). ESI-MS m / z371 ([M+1] + ,10%). Anal. Calcd for C 23 h ...
Embodiment 3
[0017] Example 3. Preparation of 2-isoamyl-3-methoxy-5-propargyloxy-stilbene (DT-3).
[0018] Weigh 0.25g (0.85mmol) of lignanin C, add anhydrous K after grinding 2 CO 3 0.1g (0.85mmol), add DMF10ml, under the protection of nitrogen, drop 0.084ml (5mmol) of 1,3-bromochloropropane, stir at 30°C for 6h, then raise the temperature to 65°C, react for 6h, filter, add acetic acid to the filtrate Ethyl ester 25ml, then extracted with 3×10mL water, anhydrous Na 2 SO 4 Dry overnight, filter, concentrate, and purify by 200-300 mesh silica gel column chromatography (petroleum ether: ethyl acetate = 18:2 as eluent) to obtain 0.23 g of white needle-like crystals (DT-3). The yield is 81.6%. m.p.130-131℃; 1 H NMR(DMSO)δ(ppm): 1.60(s,3H), 1.75(s,3H), 2.20(m,1H), 3.39(d,J=6.6Hz,2H), 3.76(s,3H), 4.22(t,J=6Hz,2H), 4.99(t,J=6.6Hz,1H), 6.53(s,1H), 6.87(s,1H), 7.13(d,J=16.2Hz,1H), 7.27 (t, J=7.2Hz, 1H), 7.36(d, J=16.2Hz, 1H), 7.37(t, J=7.8Hz, 2H), 7.50(d, J=7.2Hz, 2H). ESI-MS m / z 331 ([M-1]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com