Fatty acid compounds and preparation method and application thereof
A technology for fatty acids and compounds, applied in the field of fatty acid compounds and their preparation, can solve problems such as inability to exert normal functions, and achieve the effects of enhancing activity inhibition and promoting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 fatty acid compound
[0039] 1. Prepare the alcoholic extract of Angelica dahurica
[0040] 1) Weigh 3kg of dried Angelica dahurica root, add 30L of 60% ethanol, stir, boil and reflux for 2 hours in the extraction tank, filter, and collect the filtrate;
[0041] 2) The filter residue was boiled and refluxed with 60% ethanol to extract twice, filtered, and the filtrate was collected. The dosage of 60% ethanol was 30 L / time;
[0042] 3) Combine the filtrates for 3 times, extract with ethyl acetate, and the amount of each extractant is 30L, and collect the upper layer extract;
[0043] 4) The lower layer was extracted twice with ethyl acetate, the upper layer extracts were combined three times, and vacuum-dried to obtain 48.3 g of the alcoholic extract of Angelica dahurica (coded as: AD).
[0044] 2. Silica gel column chromatography and ODS column chromatography to prepare monomeric compounds
[0045] 1) Soak 200-300 mesh silica gel in ch...
Embodiment 2
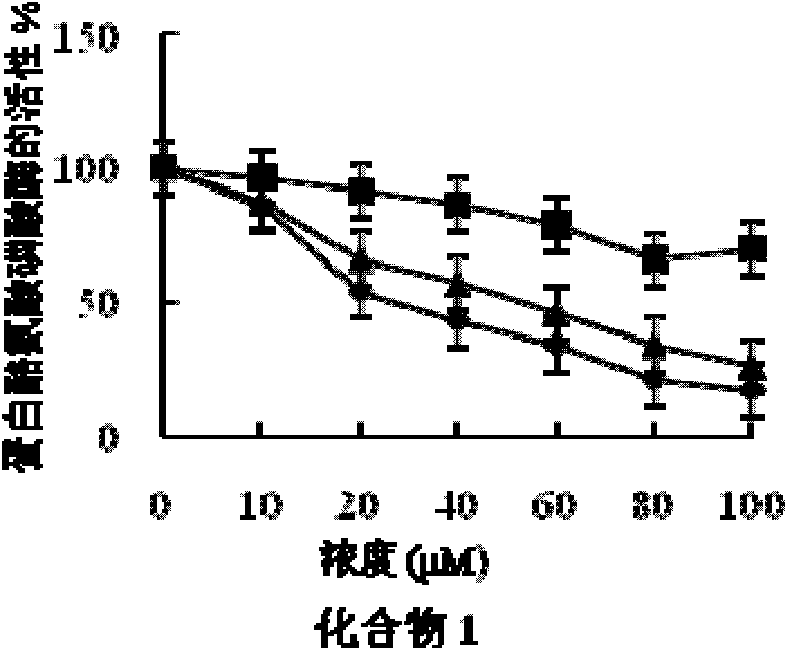
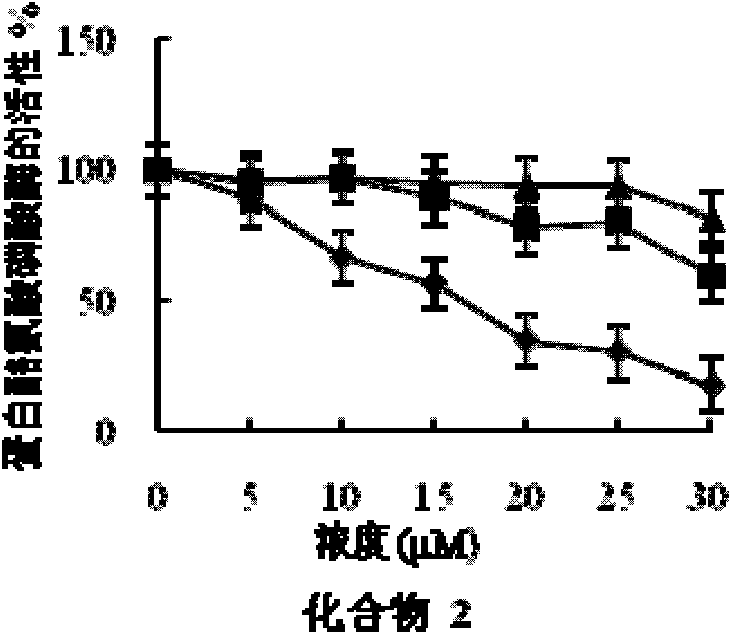
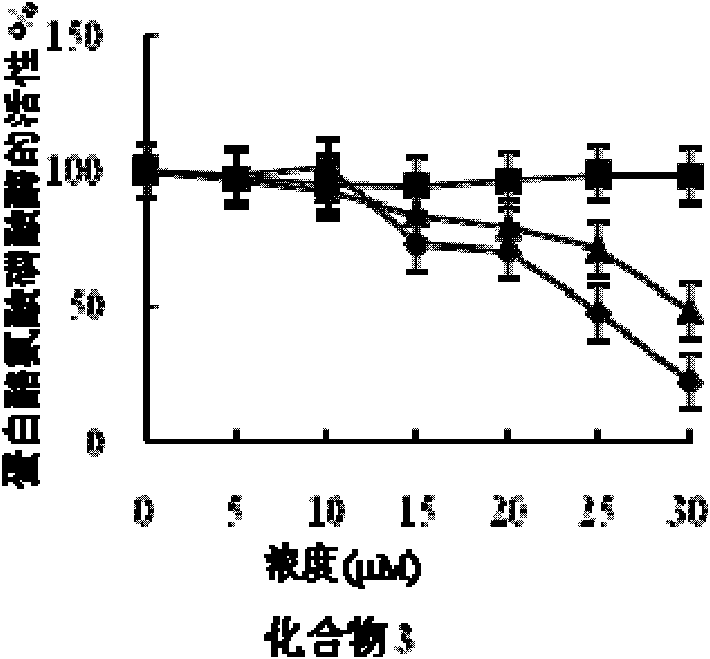
[0058] Embodiment 2 fatty acid compound pharmacodynamics experiment
[0059] 1. Experimental materials and reagents
[0060] (1) Experimental cell: human liver cancer cell HepG2
[0061] (2) Strains
[0062] pGEX4T1-PTP-SHP2 E. coli BL21
[0063] pGEX4T1-VHR E. coli BL21
[0064] pGEX4T1-HEPTP E. coli BL21
[0065] (3) Medicinal material Hangzhou Angelica dahurica produced in Zhejiang
[0066] (4) Main reagents
[0067]
[0068] (5) culture medium
[0069] LB liquid medium:
[0070] 1% (g / ml) Tryptone, 0.5% (g / ml) Yeast Extract, 1% (g / ml) NaCl
[0071] (6) Buffers and reagents required for GST purified protein
[0072] PBS Bμffer: 137mM NaCl, 2.7mM KCl, 10mM NaCl 2 HPO 4 , 2mM KH 2 PO 4 100mg / ml ampicillin
[0073] Protease Inhibitor and Phosphatase Inhibitor Stock Solutions:
[0074]
[0075]
[0076] IPTG stock solution (0.8M)
[0077] Buffer W: 25mM Tris-HCl pH7.5, 150mM NaCl, 10mM β-mercaptoethanol, 1mM EDTA
[0078] Buffer W1: 25mM Tris-HCl pH7.5...
Embodiment 314
[0196] Embodiment 314Z, the preparation of 17Z-eicosadienoic acid tablet
[0197] Get 14Z, 17Z-eicosadienoic acid 1g, microcrystalline cellulose 27g and magnesium stearate 2g and mix, and the mixture is pressed into a tablet with a diameter of 6mm and a weight of 300mg with a single-punch tablet machine. The tablet contains 10mg of 14Z, 17Z-eicosadienoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com