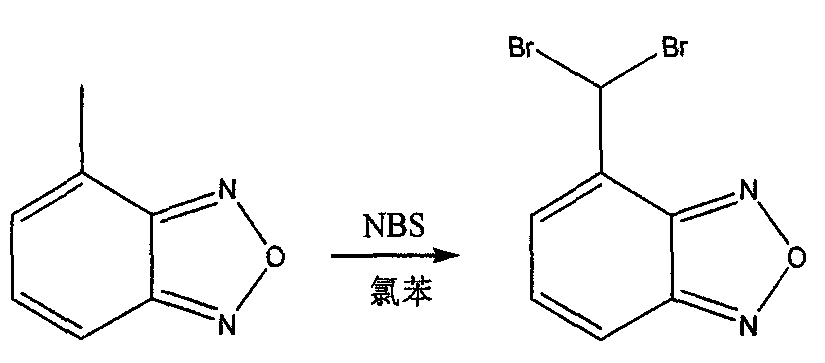
A method for preparing isradipine key intermediate 4-formylbenzofura
A technology of formylbenzene and isradipine is applied in the field of preparing isradipine key intermediate 4-formylbenzofuran, which can solve the problem of affecting the quality and yield of target product, destruction of benzfuraze, and generation of by-products. and other problems, to achieve the effect of improving yield, reducing the production of by-products, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 2
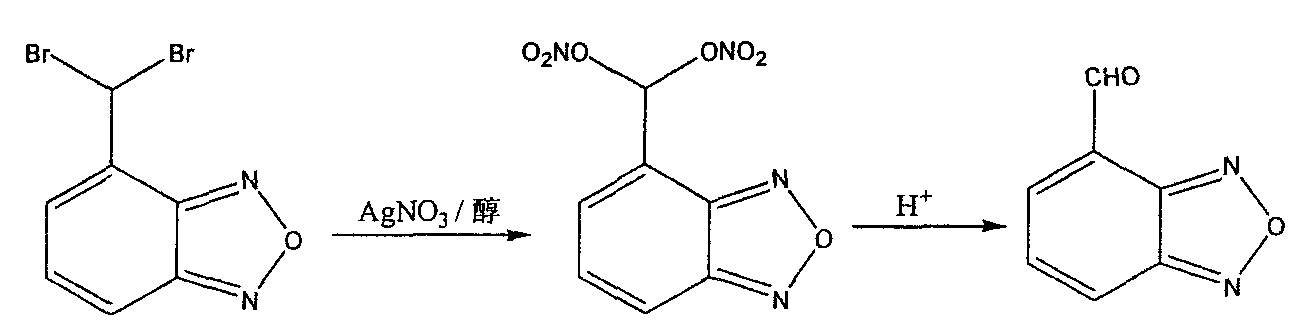
[0026] In 600ml ethanol, add 80.3g (0.275mol) 4-(dibromomethyl) benzofura, the silver nitrate solution that 187g silver nitrate and 90ml water are made into. The mixed solution was heated to 70°C, reacted for 1 hour, and filtered to remove the formed inorganic salts. 1042ml of p-toluenesulfonic acid was added to the above filtrate, and after stirring for 30 minutes, the organic solvent ethanol was distilled off. The remaining aqueous solution was diluted with 1000ml of water, and the resulting product was extracted with 400ml×2 dichloromethane. The dichloromethane solution was washed with 100ml×2 water, 80ml of 15% sodium bicarbonate solution, and 100ml of water, respectively, and dried with anhydrous sodium sulfate. The organic solvent dichloromethane was distilled off, and the remaining solid was recrystallized with cyclohexane to obtain 29.8 g of the target product with a yield of 73.1%.
specific Embodiment approach 3
[0027] In 800ml isopropanol, add 80.3g (0.275mol) 4-(dibromomethyl) benzofura, the silver nitrate solution that 187g silver nitrate and 90ml water are made into. The mixed solution was heated to 70°C, reacted for 1 hour, and filtered to remove the formed inorganic salts. 980ml of 20% hydrochloric acid solution was added to the filtrate, and after stirring for 30 minutes, the organic solvent isopropanol was distilled off. The remaining aqueous solution was diluted with 1000ml of water. The resulting product was extracted with 400ml x 2 dichloromethane. The dichloromethane solution was washed with 100ml×2 water, 80ml of 15% sodium bicarbonate solution, and 100ml of water, respectively, and dried with anhydrous sodium sulfate. The organic solvent dichloromethane was distilled off, and the remaining solid was recrystallized with cyclohexane to obtain 26.6 g of the product with a yield of 65.4%.
specific Embodiment approach 4
[0028] In 400ml methanol, add 80.3g (0.275mol) 4-(dibromomethyl) benzofura, the silver nitrate solution that 187g silver nitrate and 90ml water are made into. The mixed solution was heated to 70°C, reacted for 1 hour, and filtered to remove the formed inorganic salts. 980ml of 20% hydrochloric acid solution was added to the filtrate, and after stirring for 30 minutes, the organic solvent methanol was distilled off. The remaining aqueous solution was diluted with 1000ml of water. The resulting product was extracted with 400ml x 2 dichloromethane. The dichloromethane solution was washed with 100ml×2 water, 80ml 15% sodium bicarbonate solution, and 100ml water respectively, and dried with anhydrous sodium sulfate. The organic solvent dichloromethane was distilled off, and the remaining solid was recrystallized with cyclohexane to obtain 30.6 g of the product with a yield of 75.2%.
[0029] Description of drawings:
[0030] figure 1 It is a flow chart of the preparation met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com