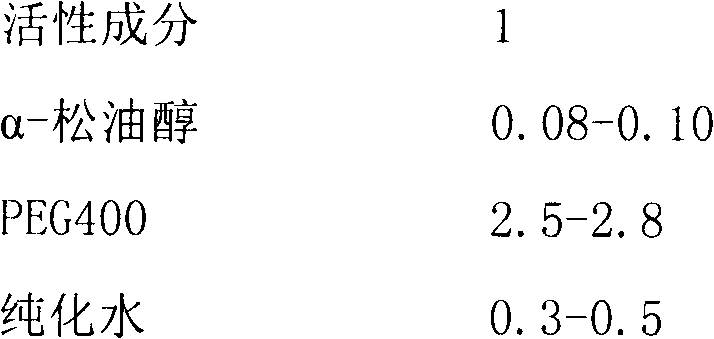Medicinal composition and soft capsule preparation for treating influenza
A composition and drug technology, applied in the direction of capsule delivery, medical preparations containing active ingredients, antiviral agents, etc., can solve the problems of capsule rupture stability, achieve stable chlorpheniramine maleate content, overcome stability Poor performance, anti-crystallization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Mix 730g of PEG400 and 75g of purified water and heat to about 60°C, add 138g of ibuprofen, 110g of zinc gluconate, 2g of chlorpheniramine maleate and 12.5g of α-terpineol, then stir slowly until dissolved to obtain color transparent solution. This transparent solution was filled into capsule shells by compression method, and each capsule shell was filled with 500 mg. Wherein, the prescription of capsule shell is shown in Table 1.
[0021] Table 1 Capsule prescription
[0022] gelatin
[0023] Through carrying out the index such as the content of ibuprofen, zinc gluconate or chlorpheniramine maleate, loading difference to the compound soft capsule prepared in embodiment 1, the result is to all meet the quality inspection standard.
Embodiment 2-5
[0025] The prescription of the capsule shell and the preparation method of the soft capsule are the same as in Example 1, and the prescription of the filling solution is shown in Table 2.
[0026] The filling liquid prescription of table 2 embodiment 2-5
[0027] components
[0028] After the compound soft capsules prepared in Examples 2-5 were tested for the contents of ibuprofen, zinc gluconate or chlorpheniramine maleate, the difference in filling capacity and other indicators, the results were all in line with the quality inspection standards.
Embodiment 6
[0032] Embodiment 6 is to the stability test research of compound soft capsule preparation
[0033] At a temperature of 37-40°C and a relative humidity of 5%, the compound soft capsule preparations prepared in Examples 1-5 of the present invention and Comparative Example 1 were placed for 3 months, and the maleic acid in the filling liquid was detected once a month. Chlorpheniramine content, the results are shown in Table 3.
[0034] Table 3 Changes in the content of chlorpheniramine maleate in the compound soft capsule preparation during the accelerated process
[0035] group
0 months
1 month
2 months
3 months
Comparative Example 1
0
-0.5%
-2.6%
-7.8%
Example 1
0
+0.4%
-0.2%
-1.4%
Example 2
0
-0.5%
-0.6%
-1.6%
Example 3
0
+0.2%
-0.3%
-1.0%
Example 4
0
+0.1%
-0.2%
-0.8%
Example 5
0
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com