Pregnenol ketopyrrole compound and preparation method thereof
A compound and drug technology, applied in the field of steroid compounds and their preparation, can solve the problems of complex yield and operation process, and achieve the effects of cheap and easily available raw materials and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
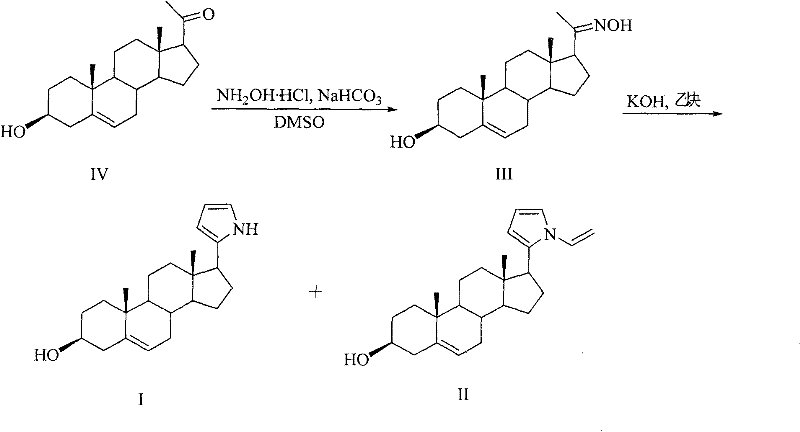
[0021] Embodiment 1: the preparation of compound I and II:
[0022] Compound IV was purchased from ACROS ORGANICS, and hydroxylamine, sodium bicarbonate, DMSO, potassium hydroxide, and acetylene were commercially available analytical reagents.
[0023]
[0024] Add 5mmol (349mg) NH in a 50ml two-necked bottle 2 OH·HCl, 10ml DMSO, then add 5mmol (421mg) NaHCO under stirring at room temperature 3 and 5 mmol (1582 mg) of pregnenolone in white powdery crystals, with a strong CO 2 Released, the solution was colorless, and a large amount of white powdery solid was suspended in the solution, and the mixture was reacted at room temperature for 5h. Then heat up to 100°C, stir, pass through acetylene under normal pressure for 0.5h, add 7.5mmol of KOH·0.5H 2 O, continue to stir at 100 ° C, with about 15 cm 3 / min speed into acetylene. After the mixture was cooled, it was diluted with water to 35ml. There were more insoluble reddish-brown massive substances at the bottom, and then...
Embodiment 2
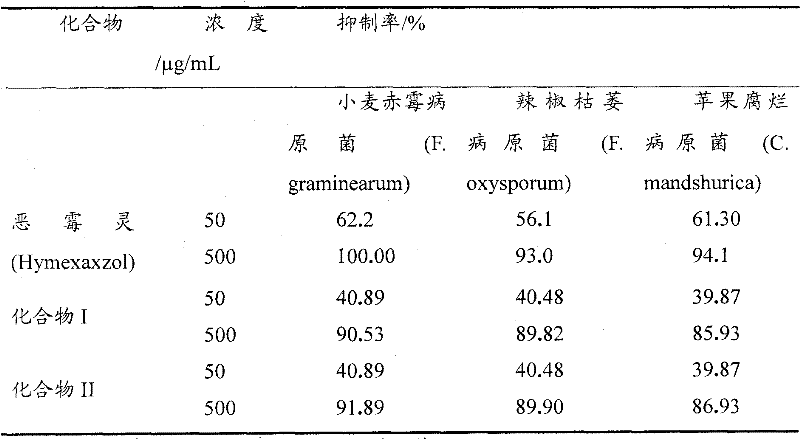
[0032] Embodiment 2: the antibacterial activity test of compound I and II
[0033] The strains to be tested were provided by the Bioassay Laboratory of the Fine Chemical Center of Guizhou University, and were purified and cultivated for later use.
[0034] Adopt growth rate method (Kong Fanbin etc., " 9 kinds of medicaments are to the indoor bacteriostasis test of maize spot bacterium ", Guangxi Agricultural Science, 2006, 37 (2): 148-149) with poisonous potato agar medium (PDA) to The target compounds were tested for their antibacterial activity against Fusarium graminearum, Fusarium oxysporum and Cytosporam and shurica.
[0035] Compounds I and II were diluted with quantitative sterile water to the desired concentration respectively, and placed in a high-speed centrifuge to rotate for 5 minutes to form a drug solution. Pour 10mL of the drug solution (10 times the final concentration of the drug solution) into 90mL of PDA in a molten state (200g of potatoes, 20g of agar, 20g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com