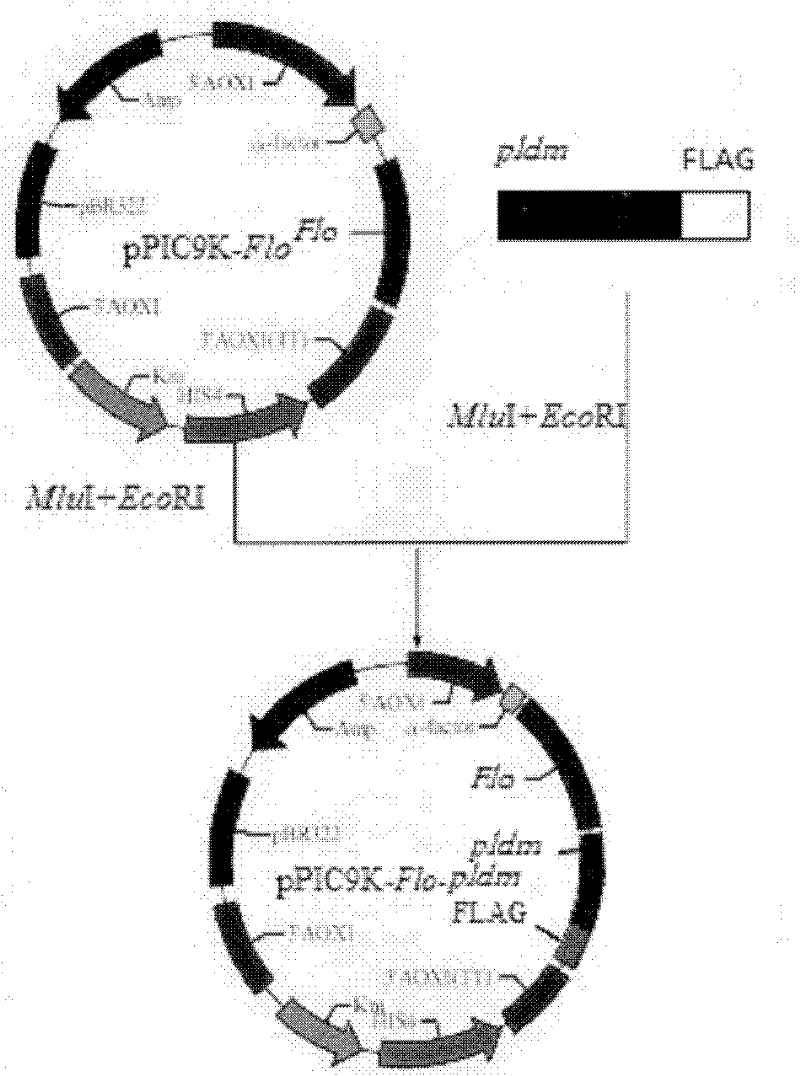
Preparation of highly active phospholipase d and yeast whole cell catalyst displaying phospholipase d on the cell surface
A whole-cell catalyst and cell surface technology, which is applied in the preparation field of high-activity phospholipase D and yeast whole-cell catalyst displaying phospholipase D on the cell surface, can solve the problem of increasing carrier cost and immobilization operation cost, low PS content, free Poor stability of phospholipase D
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] The technical contents of the present invention will be further described below in conjunction with the examples; the following examples are illustrative, not restrictive, and cannot limit the protection scope of the present invention with the following examples.
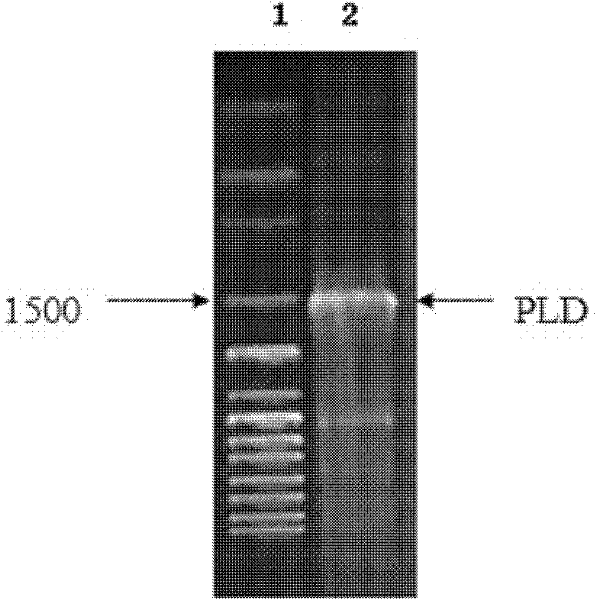
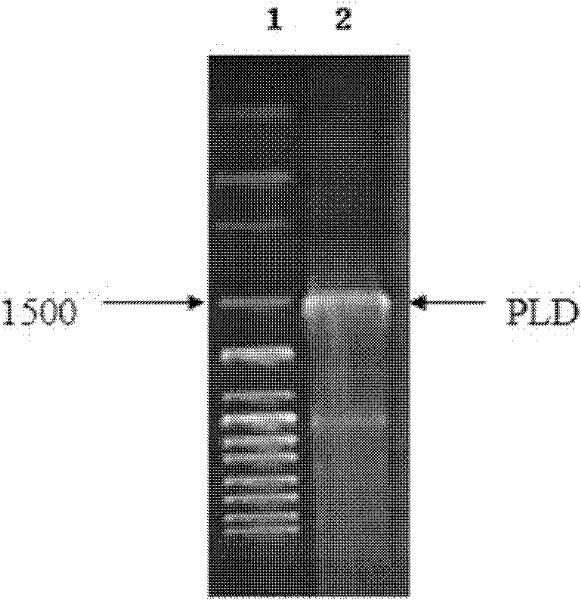
[0034] 1. Obtaining the wild-type phospholipase D mature peptide gene
[0035] 1. The wild-type phospholipase D mature peptide gene is from Streptomyces fusceus (AS 4.331), purchased from the Institute of Microbiology, Chinese Academy of Sciences, and its genomic DNA was extracted.
[0036] Wherein the extraction steps of Streptomyces chromofuscens genomic DNA are as follows:
[0037] (1) Take 1 mL of the bacterial solution cultured to the logarithmic phase, and centrifuge at 12,000 r / min for 1 min to collect the bacterial cells.
[0038] (2) Suspend the cells in 90 μL ddH2 In O, add 10 μL of 50 mg / mL lysozyme, mix well, and then keep warm in a water bath at 37 °C for 20 min.
[0039] (3) Add 400 μL of lysa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com