Amide-linked perfluoropolyether thiol compounds and processes for their preparation and use
A technology of mercaptan compound and perfluoropolyether, which is applied in the direction of mercaptan preparation, polyether coating, photoplate making process of patterned surface, etc., can solve the problems of surface depletion and depletion, and achieve cost-effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
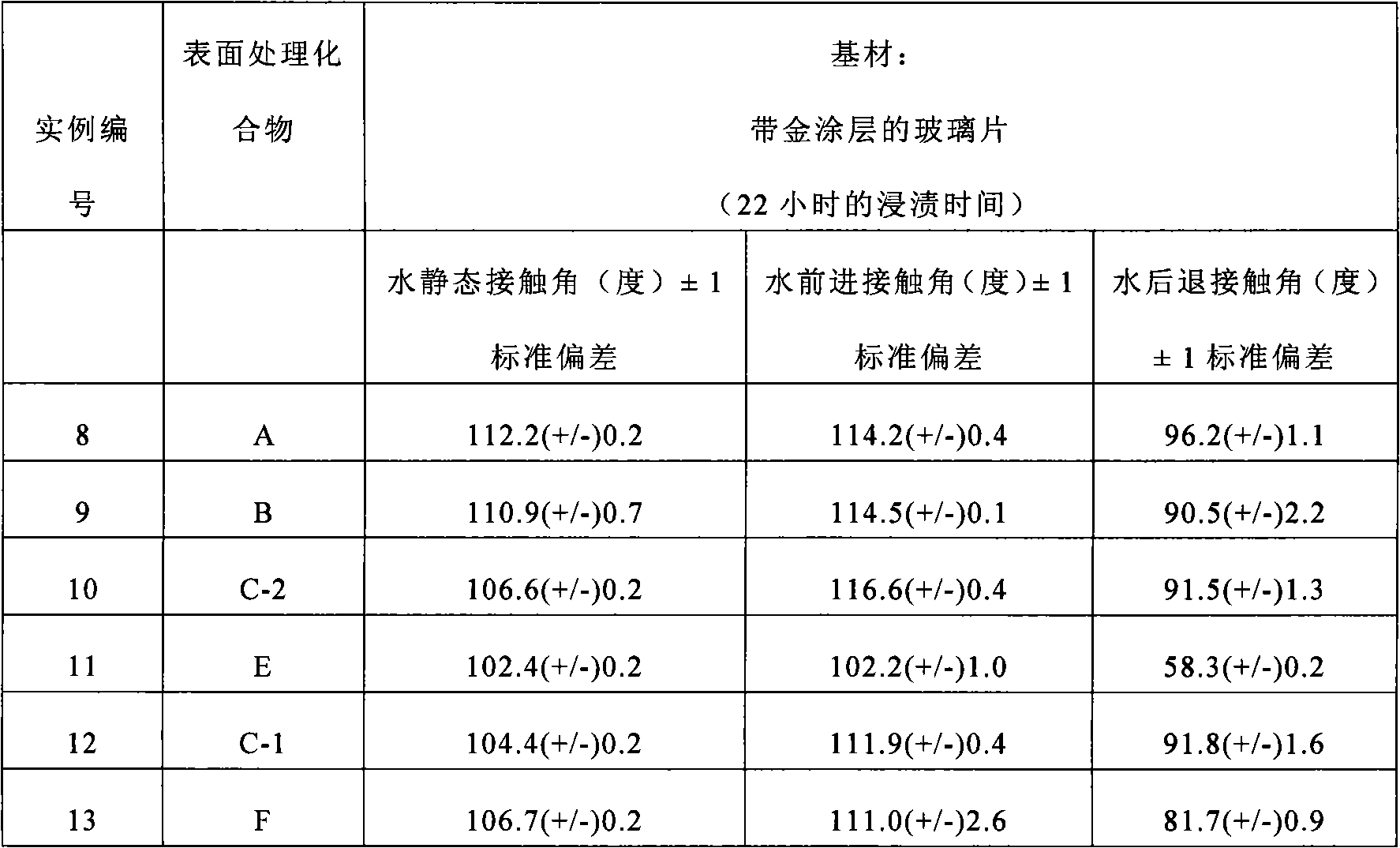
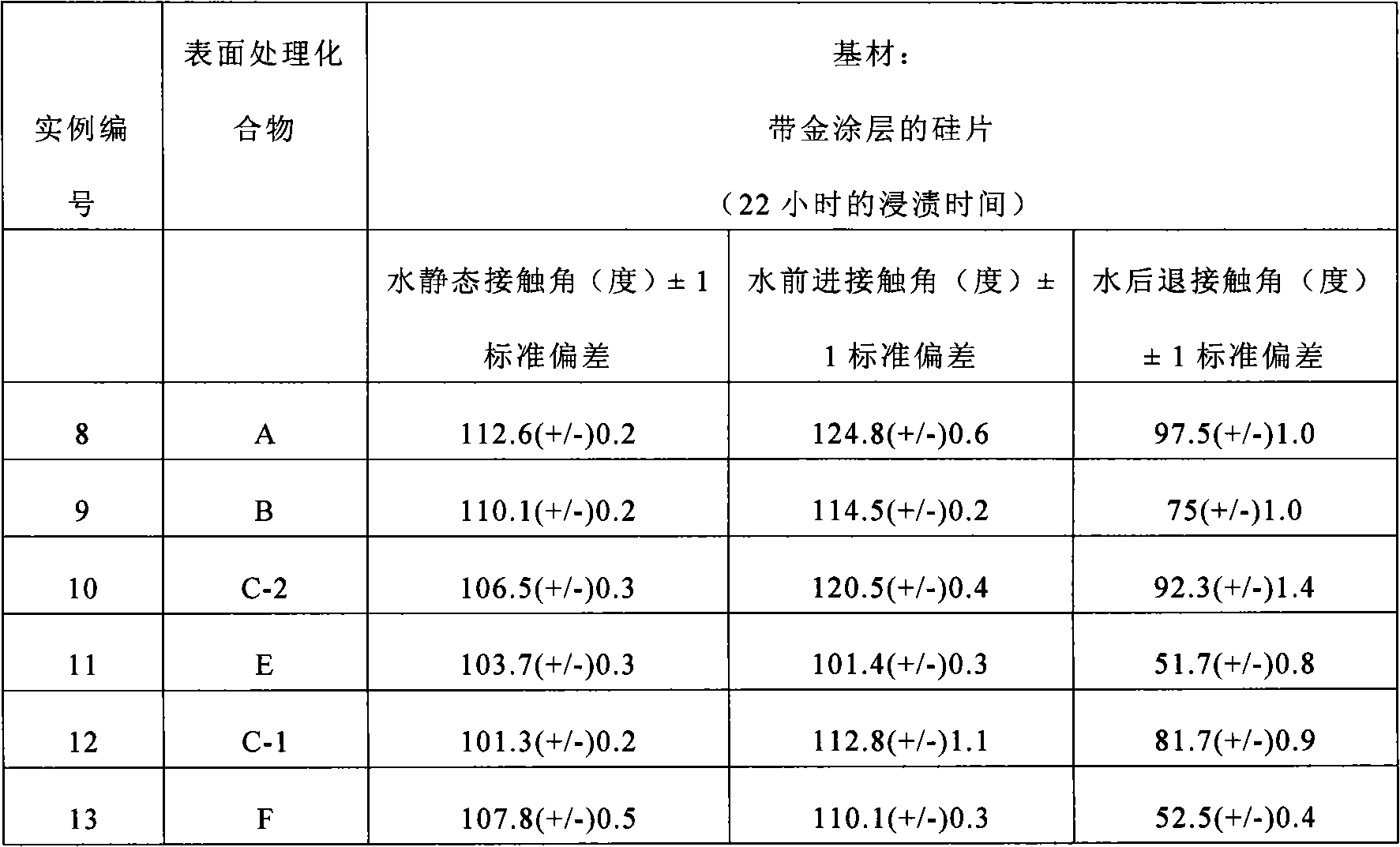
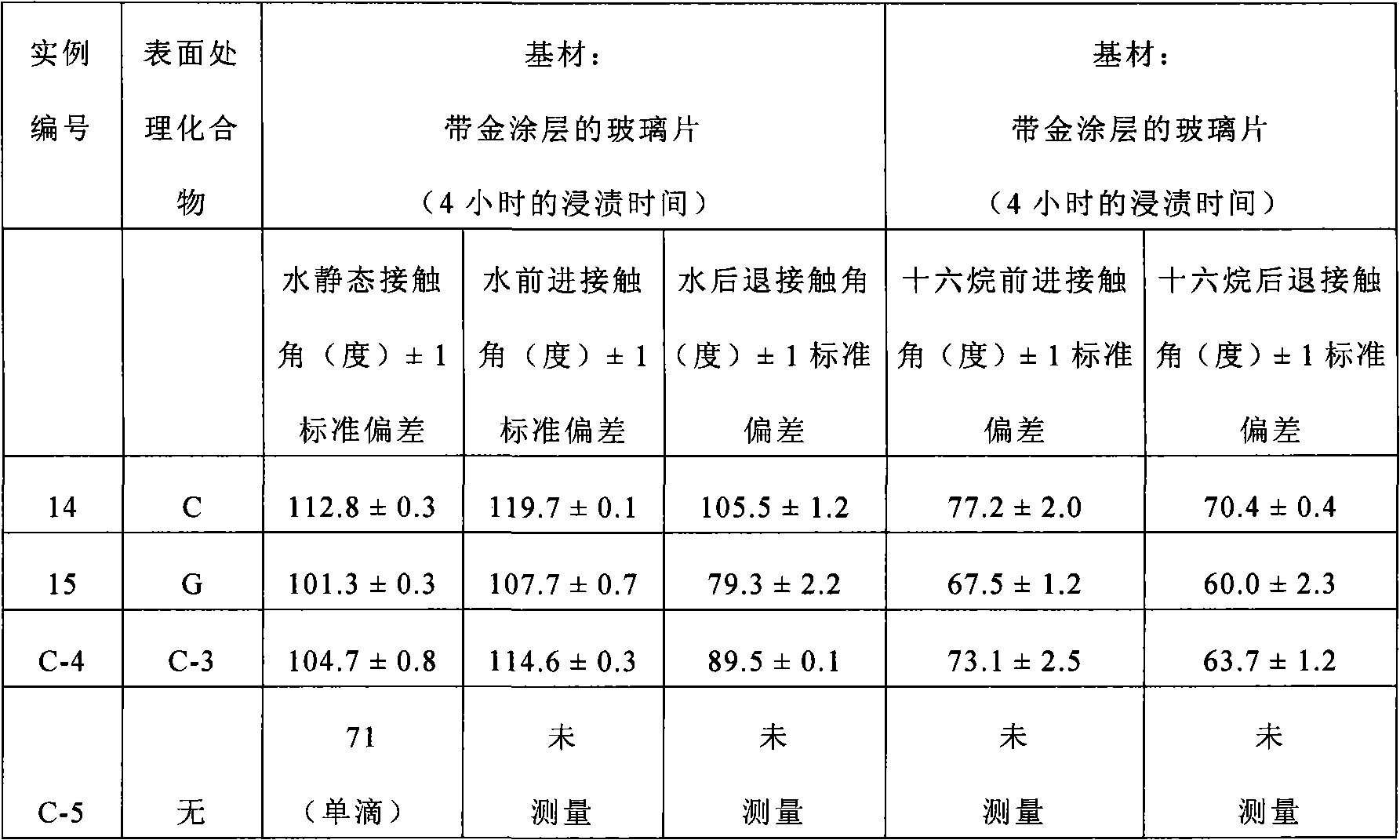
Examples
preparation example Construction
[0064] Preparation of perfluoropolyether thiol compounds
[0065] The perfluoropolyetherthiol compounds of the present invention can be prepared by various methods. For example, perfluoropolyether derivatives such as methyl esters, acid chlorides, or acid fluorides can be reacted with amine-functional alkanethiols (e.g., 2-aminoethanethiol) in water under basic conditions (e.g., NaOH or KOH). ) or the corresponding alkyl ammonium salt (for example, SH-CH 2 CH 2 -NH 3 + Cl - )reaction. However, such methods may provide complex product mixtures containing relatively low yields of the desired amide-linked perfluoropolyetherthiol compound.
[0066] Thus, the preferred method of preparation is the method of the present invention, which provides the desired compound in relatively pure form as the main product of the ring-opening reaction of the thiolactone with the corresponding amine derivative of the perfluoropolyether. The method of the present invention comprises: (a) p...
example
[0090] Objects and advantages of this invention are further illustrated by the following examples, but the particular materials and amounts thereof recited in these examples, as well as other conditions and details, should not be construed to unduly limit this invention. These examples are illustrative only, and are not intended to limit the scope of the appended claims.
[0091] Material
[0092] γ-thiobutyrolactone, mercaptoethanolammonium hydrochloride, triethylamine, N-methyl-1,3-diaminopropane, monoethanolamine, ethylenediamine, diisopropylethylamine and methanesulfonyl chloride From Aldrich Chemical Company, Milwaukee, WI. All solvents were standard reagent grade obtained from commercial sources and were used without further purification unless otherwise specified.
[0093] Unless otherwise indicated, "HFPO-" refers to the methyl ester F(CF(CF 3 ) CF 2 O) a CF(CF 3 )C(=O)OCH 3 The monovalent terminal group F(CF(CF 3 ) CF 2 O) a CF(CF 3 )-, wherein "a" averag...
example 1
[0103] HFPO-C(=O)-NH-(CH 2 ) 3 -N(CH 3 )C(=O)-(CH 2 ) 3 Synthesis of -SH (perfluoropoly Etherthiol compound A)
[0104] A 50 mL round bottom flask equipped with a magnetic stirring bar, oil bath, reflux condenser, and nitrogen inlet was charged with HFPO-C(=O)NH-CH under nitrogen atmosphere. 2 CH 2 CH 2 -N(CH 3 )H (5 g, 0.0039 moles), γ-thiobutyrolactone (4.03 g, 10 molar equivalents) and tetrahydrofuran (THF) (25 g). The resulting mixture was cloudy and it was stirred at room temperature for 5 minutes. Triethylamine (3.985 g, 10 molar equivalents) was added dropwise to the mixture via syringe at room temperature. The mixture became clear after addition of triethylamine and it was stirred at 75°C for 16 hours. Excess unreacted γ-thiobutyrolactone was distilled off under vacuum, and the resulting mixture was poured into ice water. The resulting organic phase was extracted with HFE-7100 and MgSO 4 Dry and filter. The solvent was removed under v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com