Decitabine freeze-dried preparation and preparation method thereof
A technology of decitabine and freeze-dried preparations, which is applied in the field of medicine, can solve problems such as increasing safety risks, and achieve the effects of ensuring product quality, good physical and chemical stability, and excellent comprehensive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
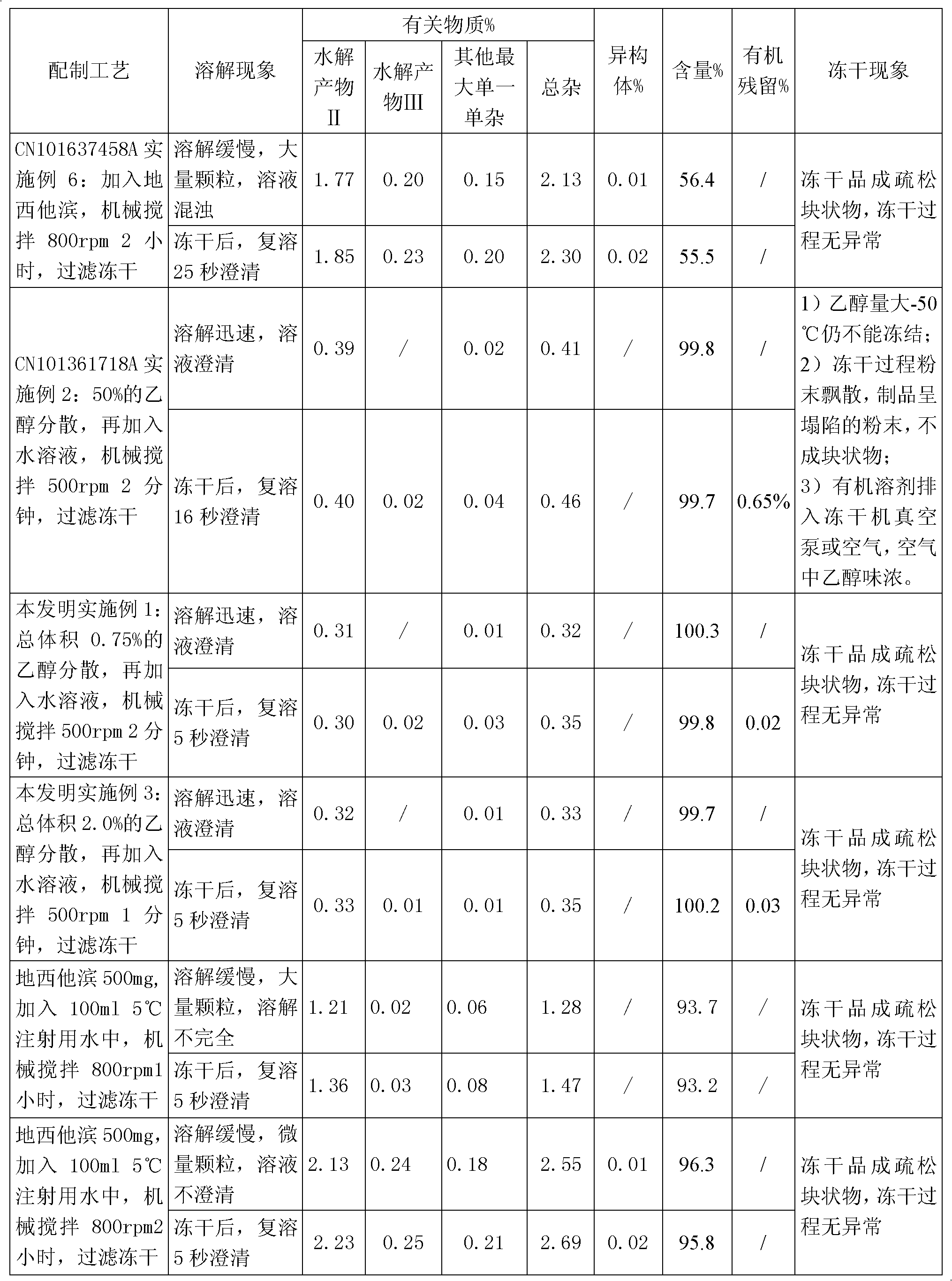
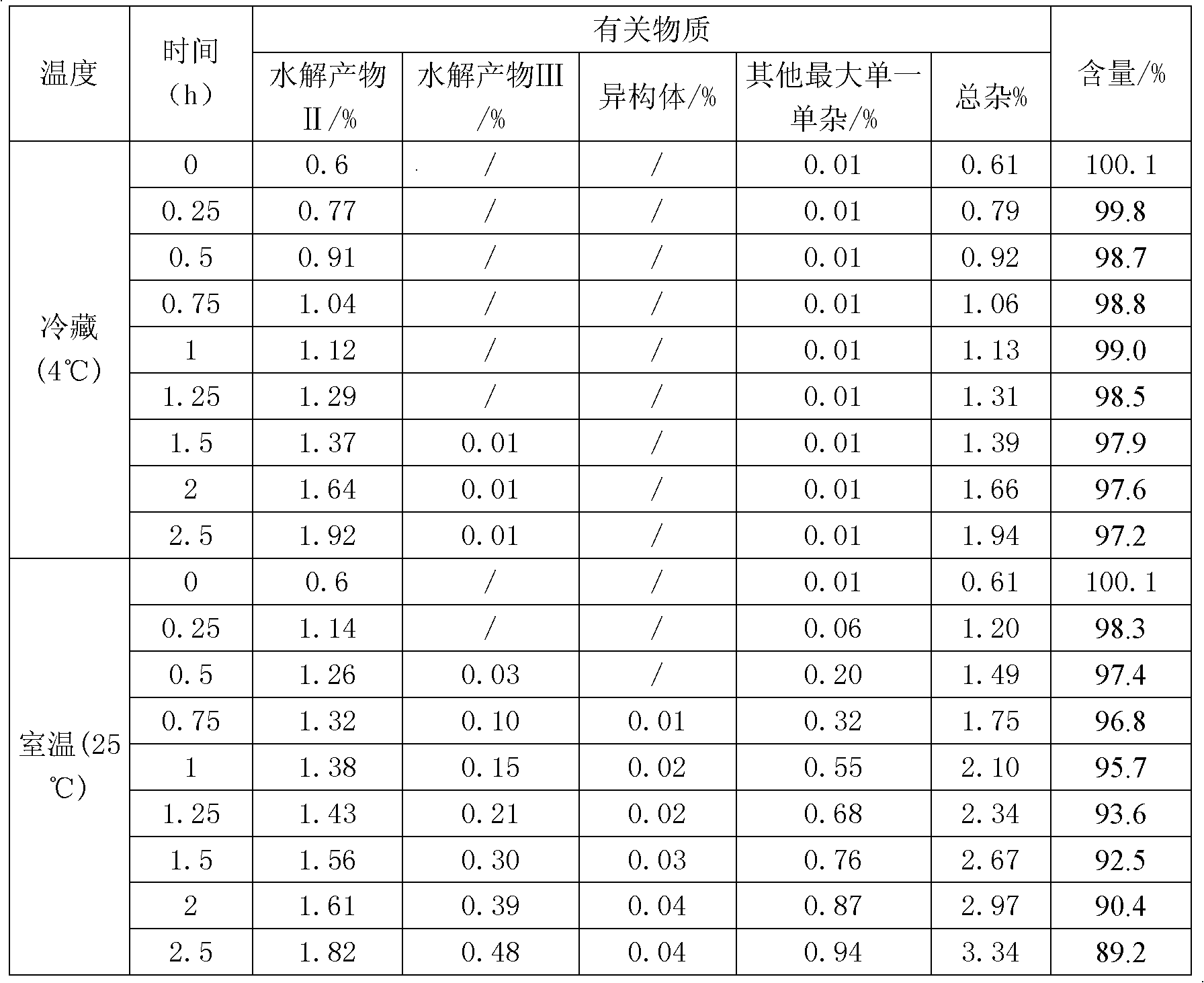
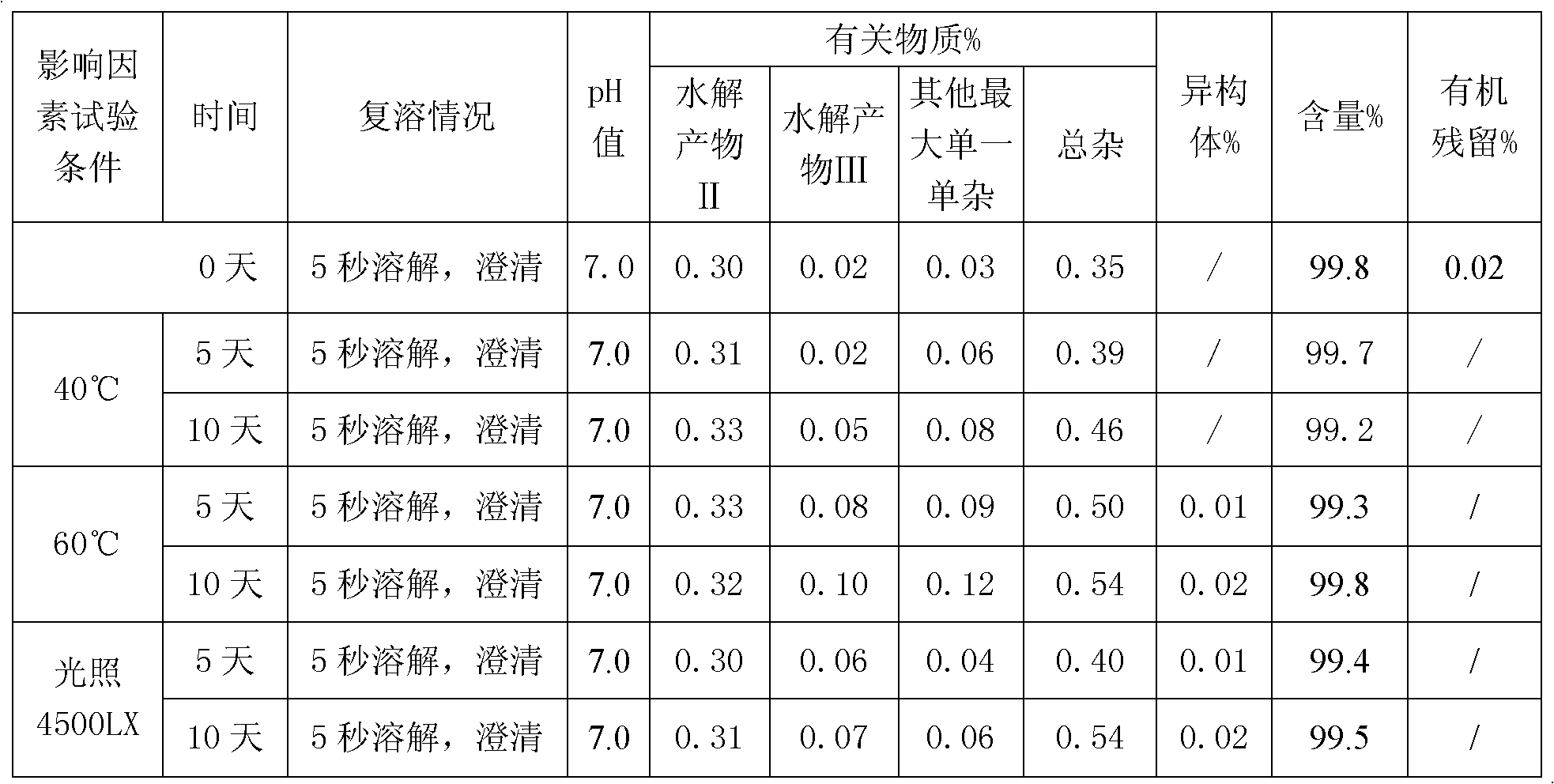
[0027] Take 9.925ml of water for injection, add 68mg of potassium dihydrogen phosphate and 11.6mg of sodium hydroxide, stir to dissolve completely, cool to 0-10°C; use it as a pre-cooled aqueous solution for later use.
[0028] Take 50 mg of decitabine in a beaker, add 0.075 ml of absolute ethanol, stir to disperse evenly, transfer to a pre-cooled aqueous solution containing potassium dihydrogen phosphate and sodium hydroxide, stir to dissolve completely, and adjust the pH of the solution to 7.0. Then use 0.45 micron and 0.22 micron filter membranes to sterilize and filter, divide into glass bottles, freeze-dry to remove ethanol and water, and fill with nitrogen to protect, stopper and cover for storage. The ethanol residue of the obtained decitabine freeze-dried preparation was 0.03%, and the related substances were 0.35%.
Embodiment 2
[0030] Take 9.95ml of water for injection, add 68mg of potassium dihydrogen phosphate and 11.6mg of sodium hydroxide, stir to dissolve completely, cool to 0-10°C as a pre-cooled aqueous solution for later use.
[0031] Take 50 mg of decitabine in a beaker, add 0.05 ml of absolute ethanol, stir to disperse evenly, transfer to a pre-cooled aqueous solution containing potassium dihydrogen phosphate and sodium hydroxide, stir to dissolve completely, and adjust the pH of the solution to 6.9. Then use a 0.22-micron filter membrane to sterilize and filter, divide into glass bottles, freeze-dry to remove ethanol and water, and fill with nitrogen to protect, stopper and cover for storage. The ethanol residue of the obtained decitabine product was 0.03%, and the related substances were 0.35%.
Embodiment 3
[0033] Take 9.8ml of water for injection, add 68mg of potassium dihydrogen phosphate and 11.6mg of sodium hydroxide, stir to dissolve completely, cool to 0-10°C as a pre-cooled aqueous solution for later use.
[0034] Take 50mg of decitabine in a beaker, add 0.2ml of absolute ethanol, stir to disperse evenly, transfer to a pre-cooled aqueous solution (0-10°C) containing potassium dihydrogen phosphate and sodium hydroxide, stir to dissolve completely, adjust the solution The pH is 7.2. Then use a 0.45 micron and / or 0.22 micron filter membrane to sterilize and filter, divide into glass bottles, freeze-dry to remove ethanol and water, and store in vacuum protection with stoppers and caps. The ethanol residue of the obtained decitabine product was 0.02%, and the related substances were 0.21%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com