Liraglutide variant and conjugate thereof
A technology for liraglutide and allosteric forms, which is applied in the field of liraglutide allosteric forms and their conjugates, can solve the problems of short half-life of polypeptide drugs, limiting patient medication compliance, poor physical and chemical stability, etc. , to achieve the effect of retaining biological activity, reducing the frequency of medication, and being difficult to degrade.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
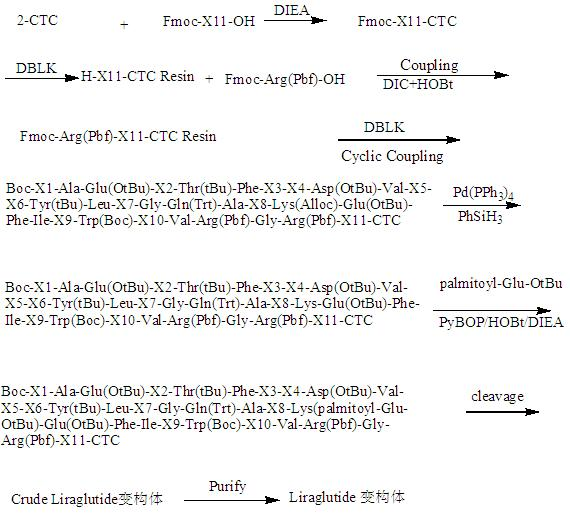
[0059] Example 1 Solid-phase synthesis of variants of liraglutide.
[0060] Using 2-chlorotrityl chloride resin (2-CTC resin) as a solid phase carrier, Fmoc protected amino groups were used to synthesize liraglutide sulfhydryl variants. The steps are as follows: Step 1: 2-CTC resin with DMF Wash 2~3 times, swell with DMF for 30 minutes; Step 2: Weigh the N-terminal Fmoc-protected amino acid, use DIEA as the activator, activate for 3~5 minutes, add the reaction column to react for 1~3 hours; Step 3: Use The mixture of piperidine and DMF with a volume ratio of 1:4 removes the Fmoc protecting group for 20 minutes. Use the ninhydrin method to detect whether the Fmoc is completely removed. The color of the resin indicates that the Fmoc has been removed; the fourth step: repeat the first step From the third step to the process, according to the amino acid sequence of the liraglutide variant, the corresponding amino acids are coupled one by one, wherein the lysine uses Fmoc-Lys(All...
Embodiment 2
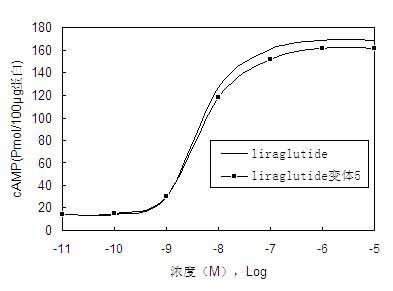
[0095] Example 2 Preparation of PEG5000 conjugate of liraglutide variant 1.
[0096] Dissolve 5 mg of liraglutide variant 1 in 8 mL of 20 mM sodium phosphate buffer (pH 6.5), and weigh 20 mg PEG5000, added to the above solution, shake properly to dissolve PEG5000 and mix well with liraglutide variant 1, react at 25°C for 2 hours, and then terminate with excess 0.5 M cysteine solution The reaction was finally placed at -20°C for separation and purification. The reaction was detected by Waters 2695 high performance liquid chromatography and purified to obtain the target compound. The molar ratio of PEG5000 to liraglutide variant 1 is 3:1, which is concluded based on the results of multiple experiments, and can completely modify liraglutide variant 1.
Embodiment 3
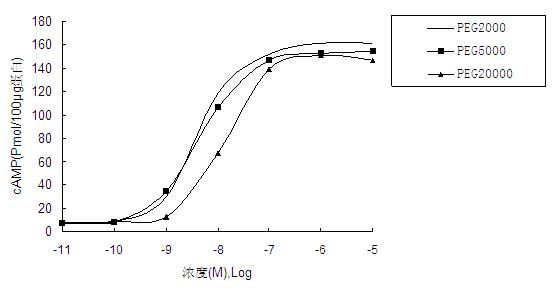
[0097] Example 3 Preparation of PEG5000 conjugate of liraglutide variant 2.
[0098] Dissolve 5 mg of liraglutide variant 2 in 8 mL of 20 mM sodium phosphate buffer (pH 6.5), and weigh 20 mg PEG5000, added to the above solution, shake properly to dissolve PEG5000 and mix well with liraglutide variant 2, react at 25°C for 2 hours, and then terminate with excess 0.5 M cysteine solution The reaction was finally placed at -20°C for separation and purification. The reaction was detected by Waters 2695 high performance liquid chromatography and purified to obtain the target compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com