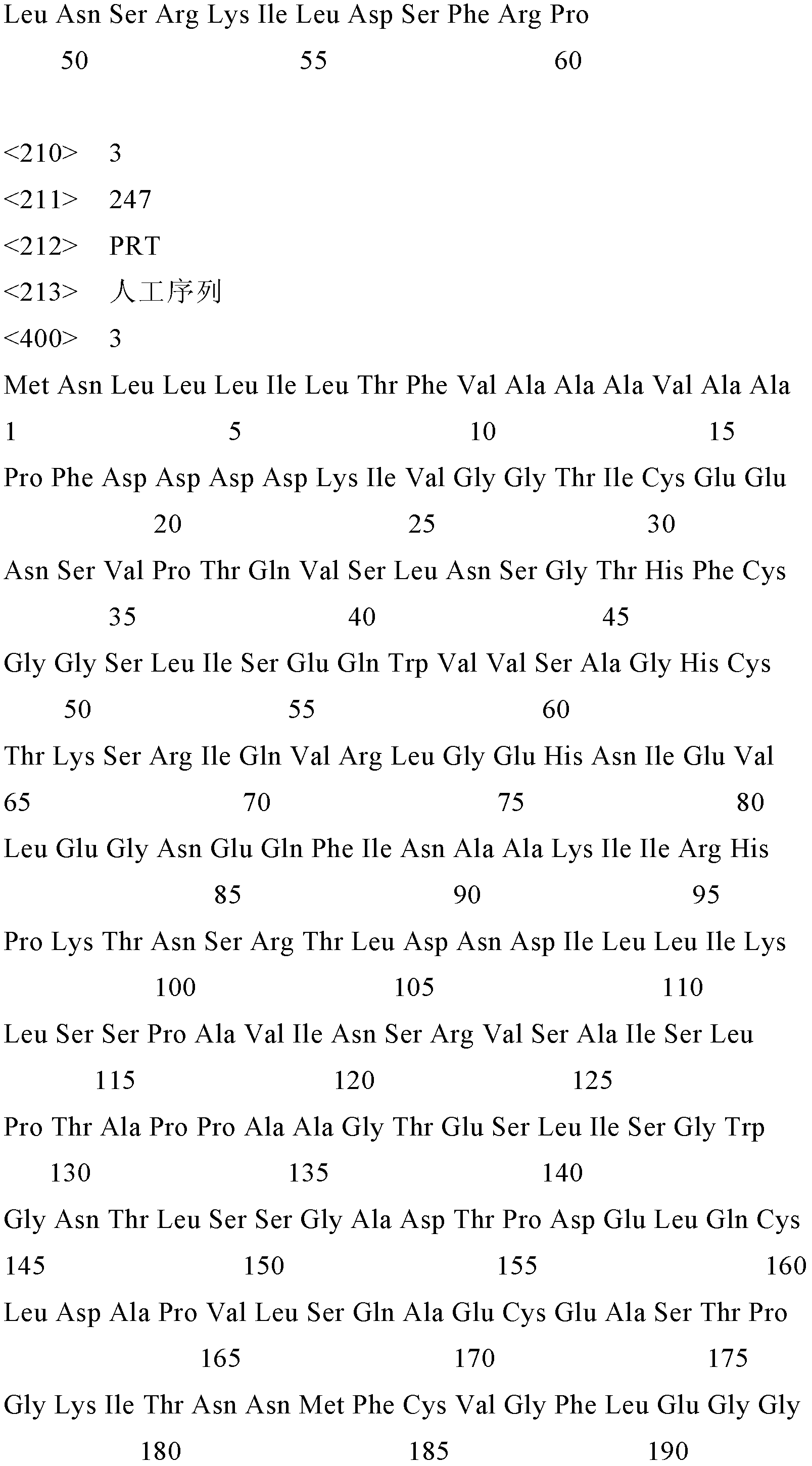Applications of beta-lactamase in improving functional protein folding and preparation of fusion protein
A technology of lactamase and fusion protein, applied in the field of fusion protein, can solve the problem that the leader sequence does not have a catalytic effect, and achieve the effect of less steps and promoting folding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Synthesis of Encoding Fusion Protein SEQ ID No.5 DNA Sequence
[0031] (1) Gene design
[0032] Using computer-aided design, the structural genes are all selected codons preferred by Escherichia coli, designed according to the amino acid sequence of SEQ ID No.5, retrieved by PC-GENE software, and adjusted to individual codons to eliminate any direct and reverse repeat sequences and Inappropriate restriction site. At the 5'-end of the structural gene, a start codon ATG is added, and a stop codon TAA, TGA and a ribosome binding site suitable for complete protein expression are added at the 3'-end.
[0033] (2) Chemical synthesis of oligonucleotide fragments
[0034] The SEQ ID No.5 gene was divided into 30 oligonucleotide fragments of 30-40mers, which were respectively synthesized on an ABI381A DNA synthesizer by the solid-phase phosphite amine method. Then, end-labeling is performed and the purity of the fragments is checked by autoradiography.
[0035] (3)...
Embodiment 2
[0039] Example 2 Synthesis of Encoding Fusion Protein SEQ ID No.6 DNA Sequence
[0040] (1) Gene design
[0041] Using computer-aided design, the structural genes are all selected codons preferred by Escherichia coli, designed according to the amino acid sequence of SEQID No.6, retrieved by PC-GENE software, and adjusted to individual codons to eliminate any direct and reverse repeat sequences and non-existent sequences. Appropriate restriction sites. At the 5'-end of the structural gene, a start codon ATG is added, and a stop codon TAA, TGA and a ribosome binding site suitable for complete protein expression are added at the 3'-end.
[0042] (2) Chemical synthesis of oligonucleotide fragments
[0043] The gene of SEQ ID No.6 was divided into 10 oligonucleotide fragments of 28-35mers, which were respectively synthesized on the ABI381A DNA synthesizer by the solid-phase phosphite amine method. Then, end-labeling is performed and the purity of the fragments is checked by auto...
Embodiment 3
[0048] Preparation of Trypsinogen in the Existence of Example 3 Leader Sequence
[0049] (1) Acquisition of DNA and Construction of Bacterial Expression Vectors
[0050] A DNA fragment encoding the amino acid sequence of SEQ ID No.5 was chemically synthesized by the solid-phase phosphite amine method (see Example 1 for the specific method). The synthetic DNA fragment contains a fragment from 5' end Cla I to 3' end Kpn I and a fragment from 5' end Kpn I to 3' end Hind III, and they are respectively cloned into the pUC vector, wherein Kpn I can cut the coding Nucleic acid sequence of amino acid residues 51 and 52 of SEQ ID No.5. Then the DNA fragment encoding the complete amino acid sequence of SEQ ID No.5 was subcloned into the modified pATH2 vector, so that the expression of the fusion protein was regulated by the Trp promoter and the SD sequence. The obtained vector pTRY-1 is used for the expression of BL-trypsinogen fusion protein.
[0051] (2) Expression of BL-trypsinoge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Final concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com