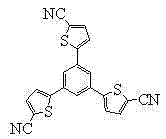
Star-shaped oligothiophene derivative, preparation method and application thereof
A technology of thiophene derivatives and thiophene groups is applied in the field of star-shaped oligomerized thiophene derivatives and the preparation thereof, which can solve the problems of unsuitable flexible processing, high manufacturing cost, non-degradation and the like, achieves good chemical and environmental stability, and is not easy to form. Effects of Film, Oxidation State and Reduction State Stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1) Preparation of 3TB-2CHO:
[0043] In the first step, under the protection of nitrogen, add N , N -Dimethylformamide, slowly dropwise adding phosphorus oxychloride, controlling the reaction temperature to 30°C, stirring and reacting for 65 minutes to obtain a reaction solution;
[0044] In the second step, 1,3,5-tri(2 ' -thienyl)-benzene, stirred and reacted for 35 minutes to obtain a reaction solution;
[0045] In the third step, after the reaction is over, add distilled water to the reaction solution obtained in the second step for hydrolysis, and the obtained flocculent precipitate is the crude product of 3TB-2CHO, vacuum filter out the flocculent precipitate, and then use a chromatographic column to filter the flocculent precipitate Isolation was performed to obtain the pure product of 3TB-2CHO.
[0046] The structural characterization data of 3TB-2CHO are as follows:
[0047] Mass spectrum: m / z 380.9 (M+);
[0048] Proton NMR: δ(ppm): 9.96(s, 2H), 8.15~8.0...
Embodiment 2
[0066] 1) Preparation of 3TB-2CHO:
[0067] The first step, under the protection of nitrogen, was added to the three-necked flask N , N -Dimethylformamide, slowly drip phosphorus oxychloride, control the reaction temperature to 45 ° C, and stir the reaction for 40 minutes to obtain a reaction solution;
[0068] In the second step, add 1,3,5-tri(2 ' -thienyl)-benzene, stirred and reacted for 30 minutes to obtain a reaction solution;
[0069] The third step, after the reaction is completed, add distilled water to the above-mentioned reaction solution for hydrolysis to obtain a flocculent precipitate, which is the crude product of 3TB-2CHO by vacuum filtration, which is then separated by a chromatographic column to obtain 3TB-2CHO. pure product.
[0070] The structural characterization data of 3TB-2CHO are as follows:
[0071] Mass spectrum: m / z 380.9 (M+);
[0072] H NMR spectrum: δ(ppm): 9.96(s, 2H), 8.15~8.05(m, 4H), 8.03(s, 3H), 7.85(d, 1H), 7.71(d, 1H), 7.25(t) , 1H)...
Embodiment 3
[0090] 1) Preparation of 3TB-2CHO:
[0091] The first step, under the protection of nitrogen, was added to the three-necked flask N , N -Dimethylformamide, slowly drip phosphorus oxychloride, control the reaction temperature to 65 ° C, and stir the reaction for 20 minutes to obtain a reaction solution;
[0092] In the second step, add 1,3,5-tri(2 ' -Thienyl)-benzene, stirred and reacted for 35 minutes to obtain a reaction solution;
[0093] In the third step, after the reaction is completed, distilled water is added to the reaction solution obtained in the second step for hydrolysis to obtain a flocculent precipitate, and the flocculent precipitate is filtered out by vacuum suction, which is the crude product of 3TB-2CHO, which is then separated by a chromatographic column. The pure product of pure 3TB-2CHO was obtained.
[0094] The structural characterization data of 3TB-2CHO are as follows:
[0095] Mass spectrum: m / z 380.9 (M+);
[0096] H NMR spectrum: δ(ppm): 9.96...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com