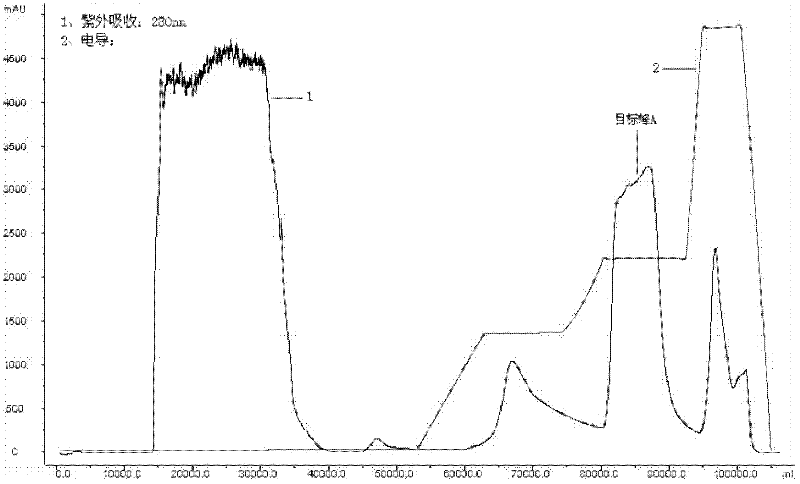
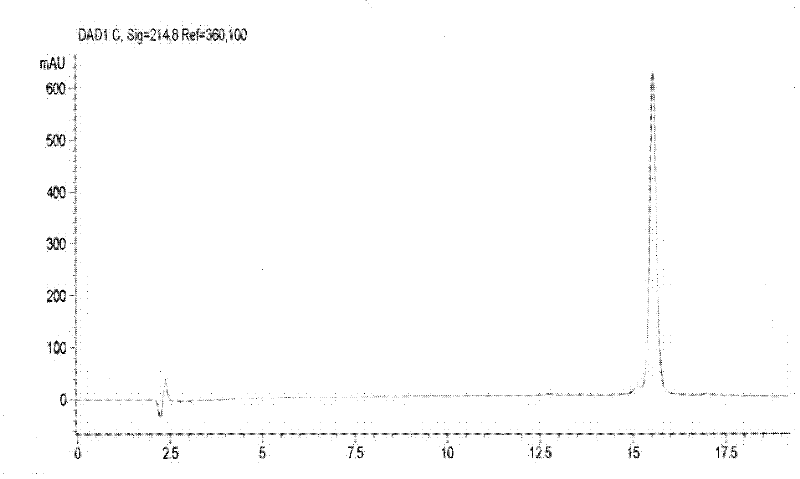
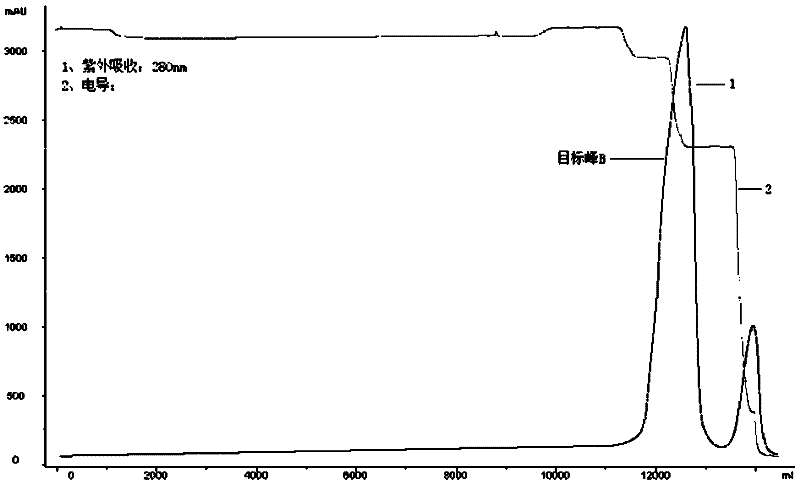
Production method for Pichia pastoris expression recombinant human interleukin 11
A technology for Pichia pastoris and human interleukin, which is applied in the field of large-scale purification of rhIL-11, can solve the problems of low expression amount, high production cost, unfavorable purification and the like, achieves simple operation, reduces pollution and loss, and is beneficial to large-scale the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The engineered strain Pichia pastoris JYIL4 (CCTCC NO: M 204072) is fermented (refer to the published patent, application number 99118279.0). The present invention adopts two-step fermentation. In the first stage, the engineered bacteria are inoculated in a semi-synthetic medium with carbon sources such as glycerol or glucose (the formula is: sodium hexametaphosphate 25g / L, CaSO 4 ·2H 2 O 1.176g / L, K 2 SO 4 18.2g / L, MgSO 4 7.28g / L, EDTA 0.925g / L, glycerol 40g / L, PTM1 4.35ml / L, (NH 4 ) 2 SO 4 9g / L, bubble enemy 0.02%. The formula of PTM1 is: CuSO 4 ·5H 2 O 6g / L, NaI 0.08g / L, MnSO 4 ·2H 2 O 3g / L, Na 2 MoO 4 ·2H 2 O 0.2g / L, boric acid 0.02g / L, biotin 0.2g / L, CoCl 2 0.5g / L, ZnCl 2 20g / L, FeSO 4 ·7H 2 O 65g / L, sulfuric acid 5ml) incubate for 16-28 hours until glycerol or glucose is exhausted, and the bacterial density reaches greater than OD 600 =160, this is the growth period of the bacteria. In the second stage, methanol is fed to a concentration of 0.5%-5% to induce ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com