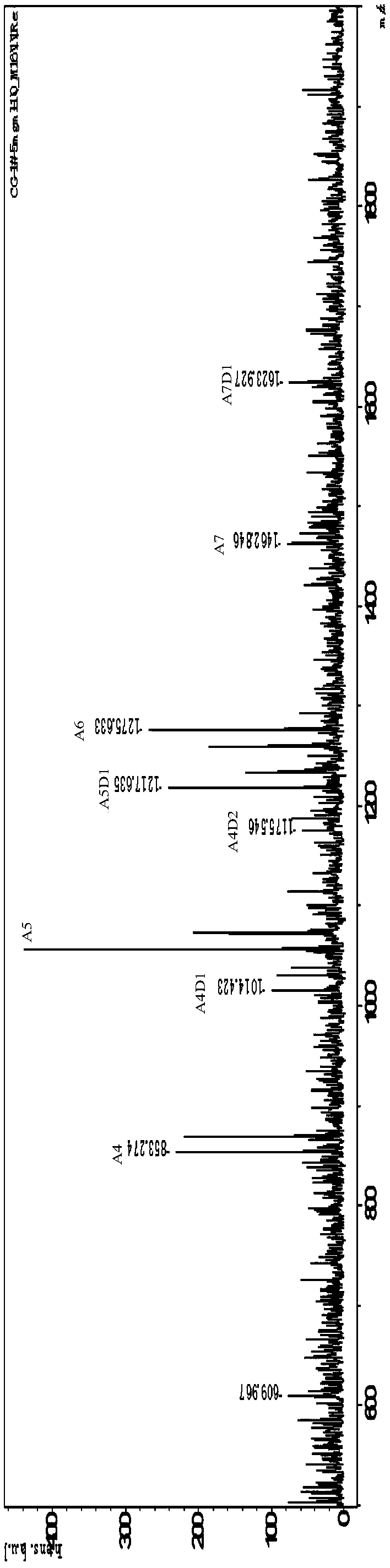
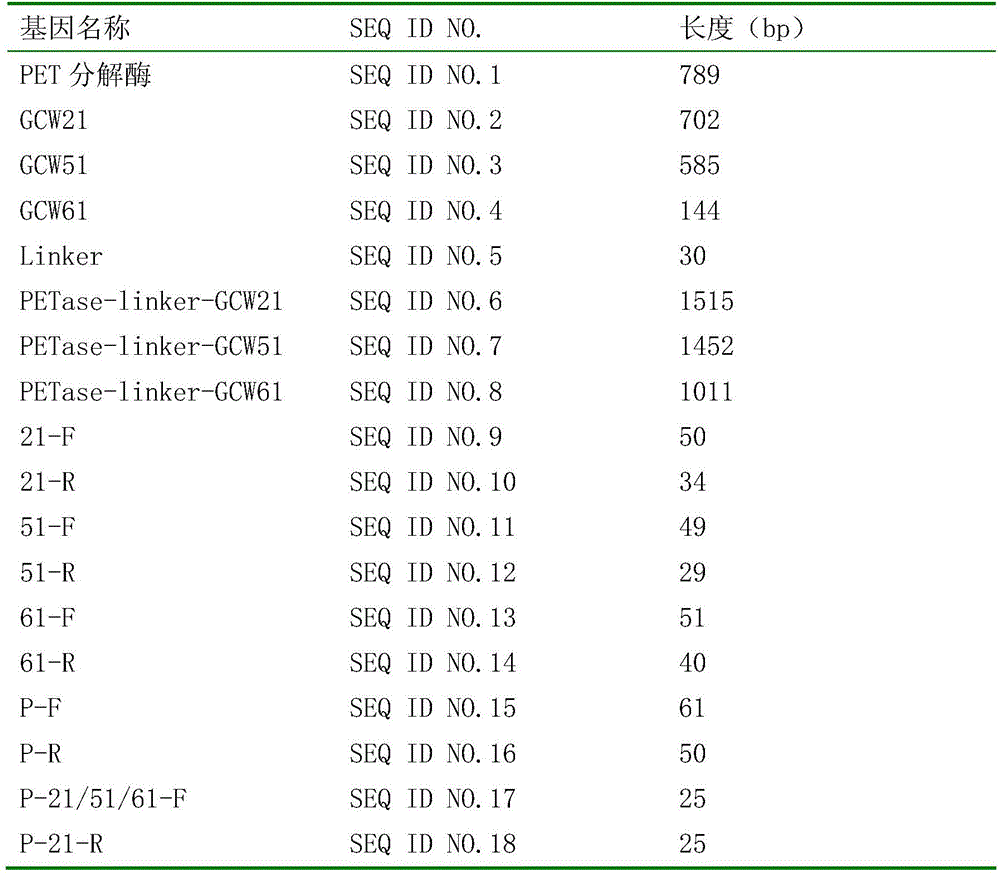
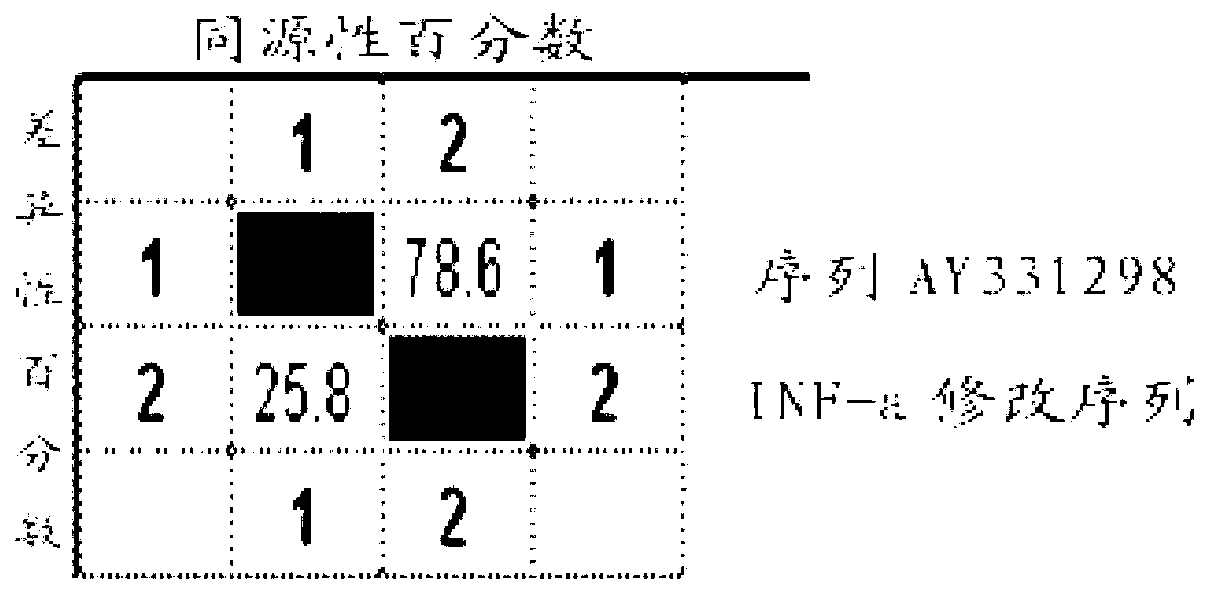
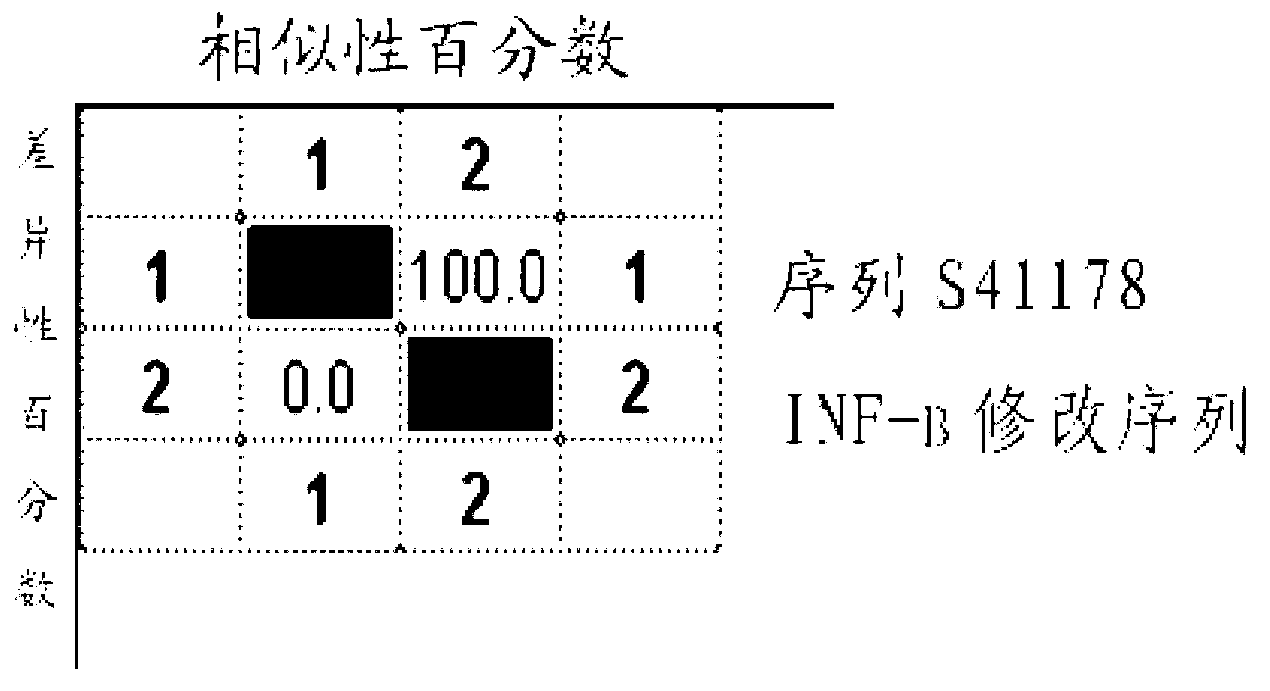
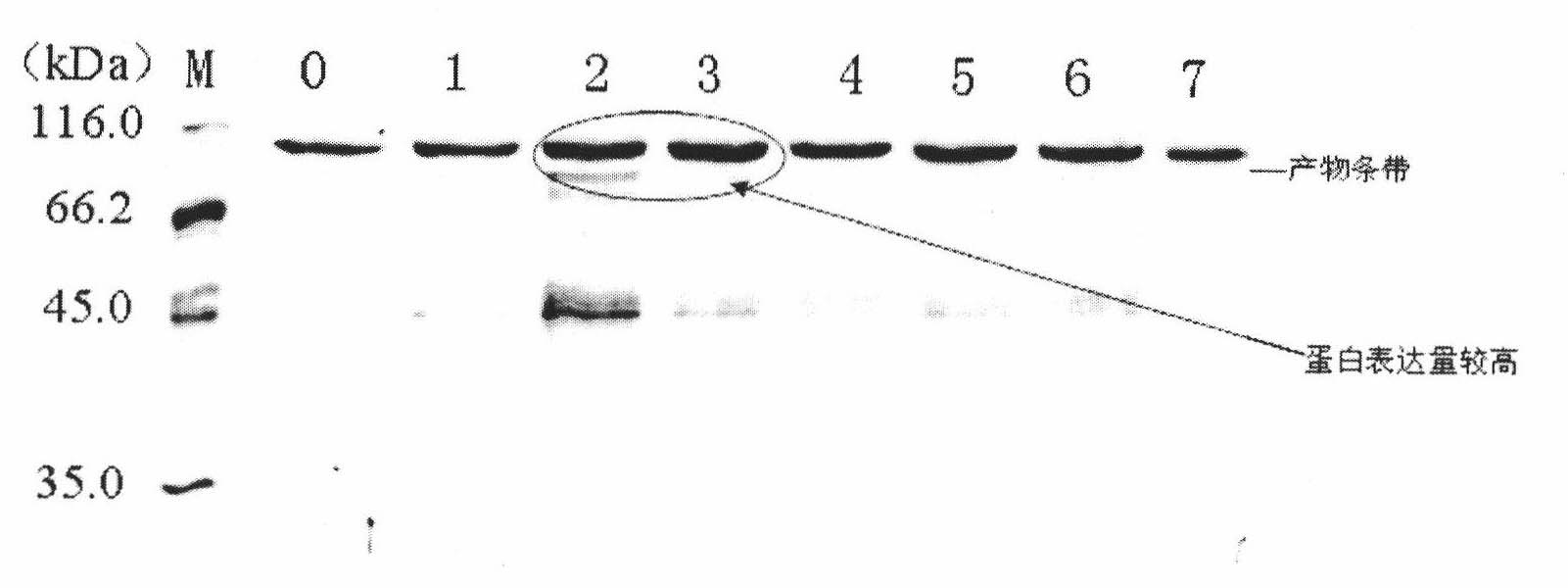
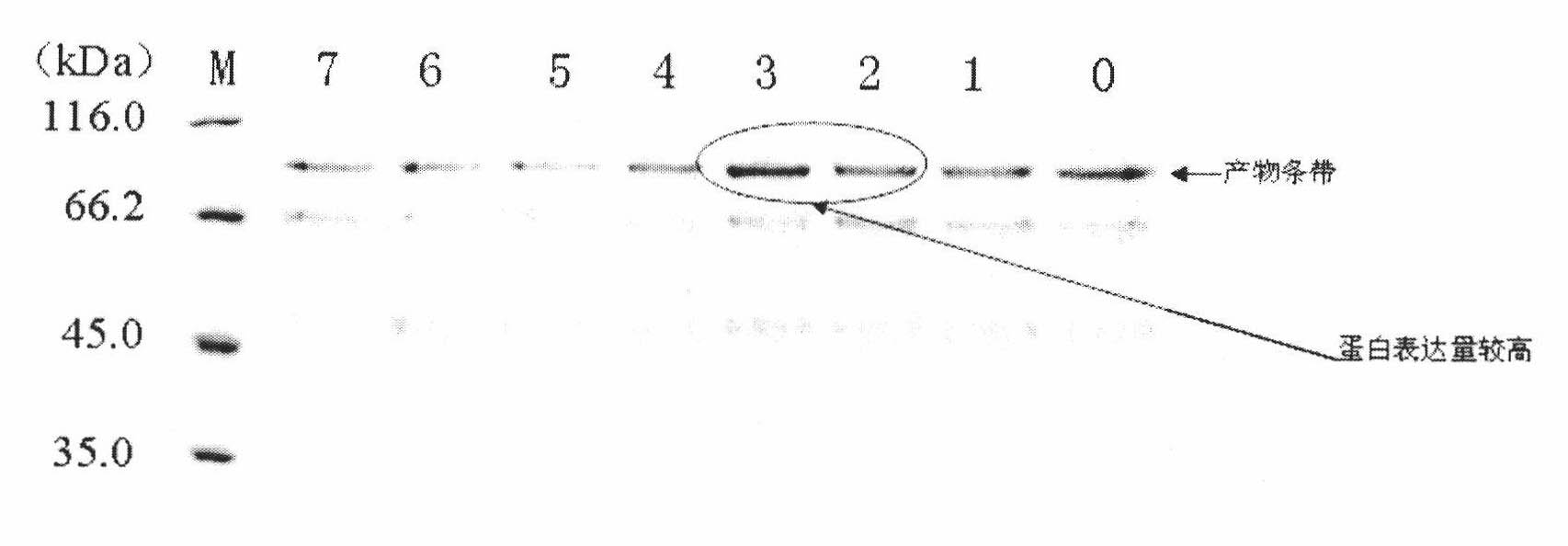
Patents
Literature
104 results about "Pichia ambrosiae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fig. l. Pichia ambrosiae v.d. Walt et Scott. (a) Vegetative cells in malt extract after 3 days at 25 C. (b) Anastomosing hyphae on corn meal agar after 7 days at 25 C. (c) Early stages of development of the ascophoric hyphae. (d) Mature ascophoric hyphae with asci and ascospores.
Recombinant pichia pastoris for heterogenous high level expression of lipase
InactiveCN103361327AImprove expression efficiencyReduce the cost of separation and purificationFungiHydrolasesPichia pastorisHeterologous
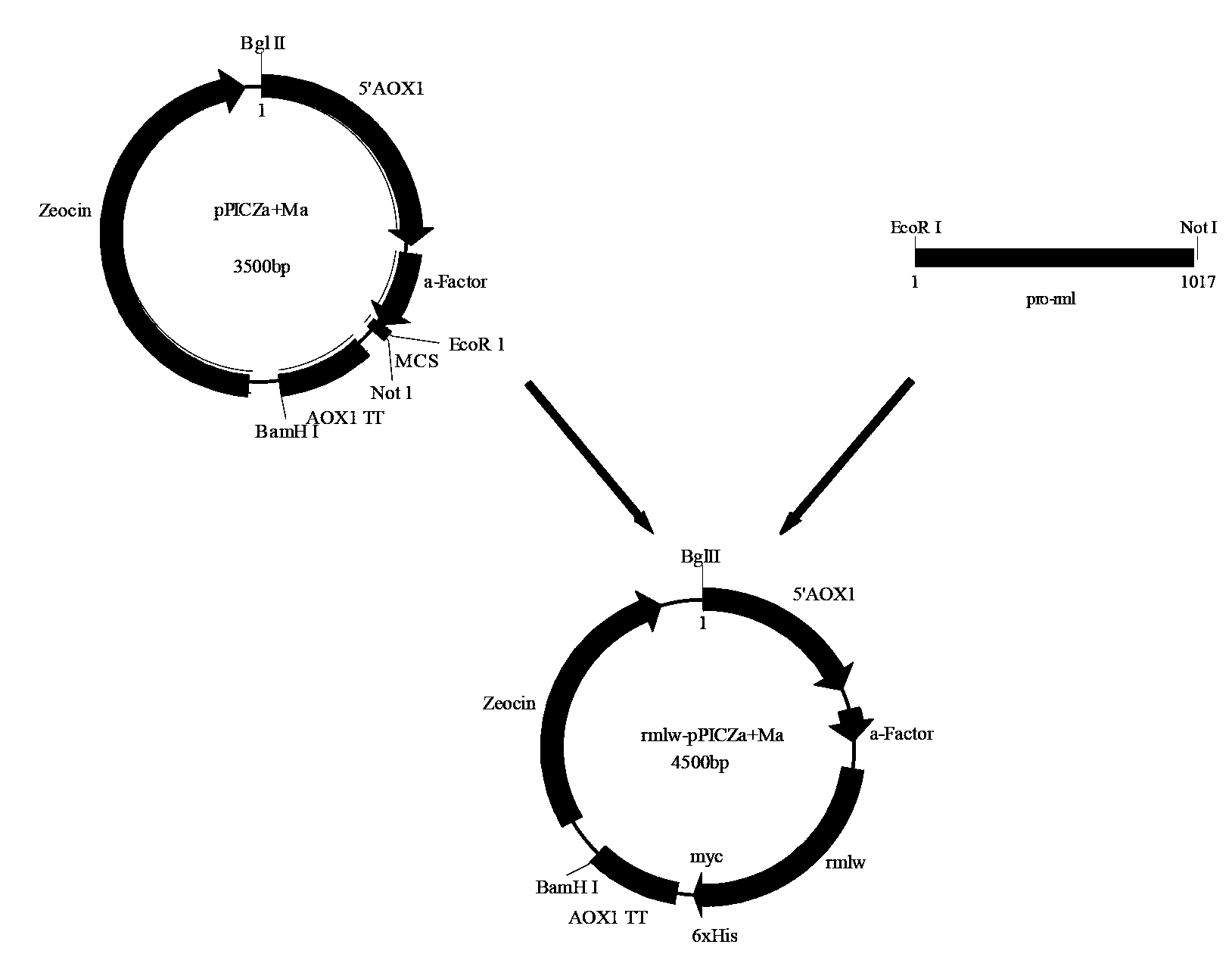
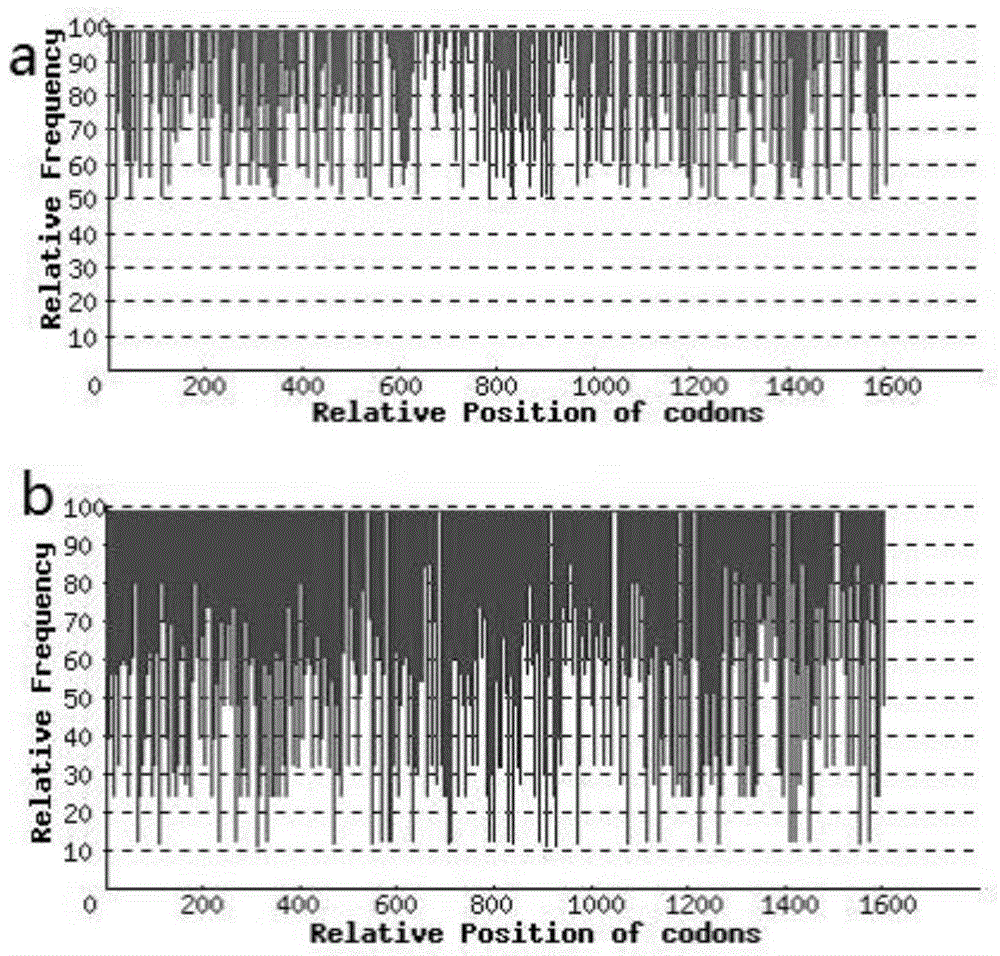
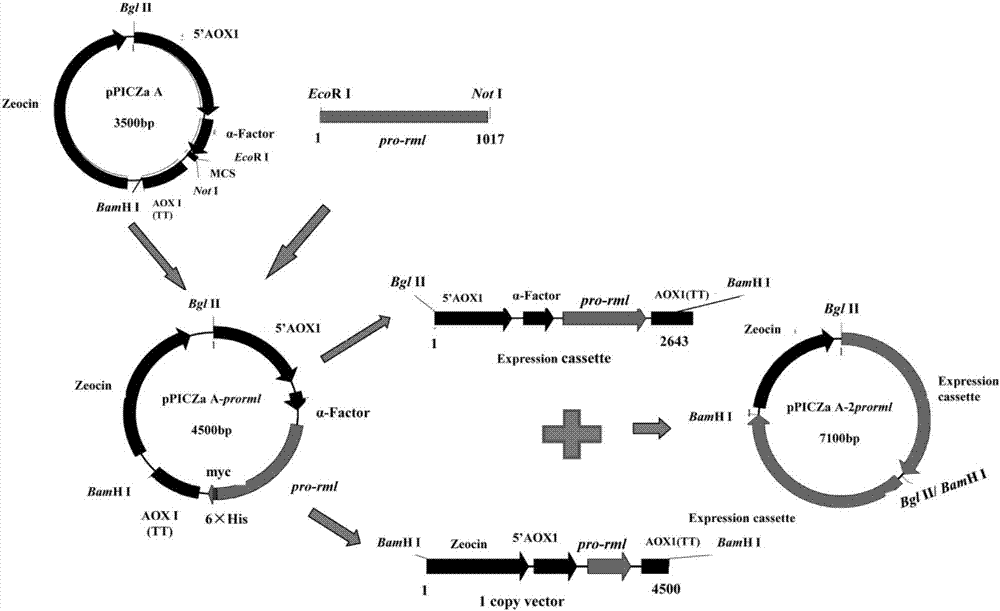
The invention discloses recombinant pichia pastoris for heterogenous high level secretory expression of a rhizomucor miehei lipase. The recombinant pichia pastoris is obtained by introducing a self leader peptide gene for encoding the rhizomucor miehei lipase by employing a molecular biology technology, then optimizing a copy number of an objective gene, and constructing a vector which can be expressed in pasteur pichia pastoris. Researches show that: the introduction of the self leader peptide greatly increases the secretion amount of the objective lipase, a pichia pastoris recombinant of a lipase gene (pro-rml) containing two copies has highest enzyme activity, and the lipase Pro-RML has higher activity of hydrolysis of tri-acylglycerol.
Owner:CHINA AGRI UNIV
Pichia yeast YPD-YL2 and application of same in biological deodorization
The invention provides a Pichia yeast YPD-YL2 and application of the same in biological deodorization. The Pichia yeast YPD-YL2 has a Latin name of Pichia sp. and an accession number of CCTCC M 2015225. The Pichia yeast YPD-YL2 provided by the invention is separated from an environment of a refuse landfill, is capable of removing foul gas produced by garbage decomposition and can decompose garbage and produce slightly smelly gas. Repeated addition of the Pichia yeast YPD-YL2 is not needed in deodorization with the Pichia yeast YPD-YL2, so workload is mitigated. Due to security of the Pichia yeast YPD-YL2, the Pichia yeast YPD-YL2 can also be used for treating substances like animal feeds and additives so as to allow the substances to be edible to animals.
Owner:杨利平
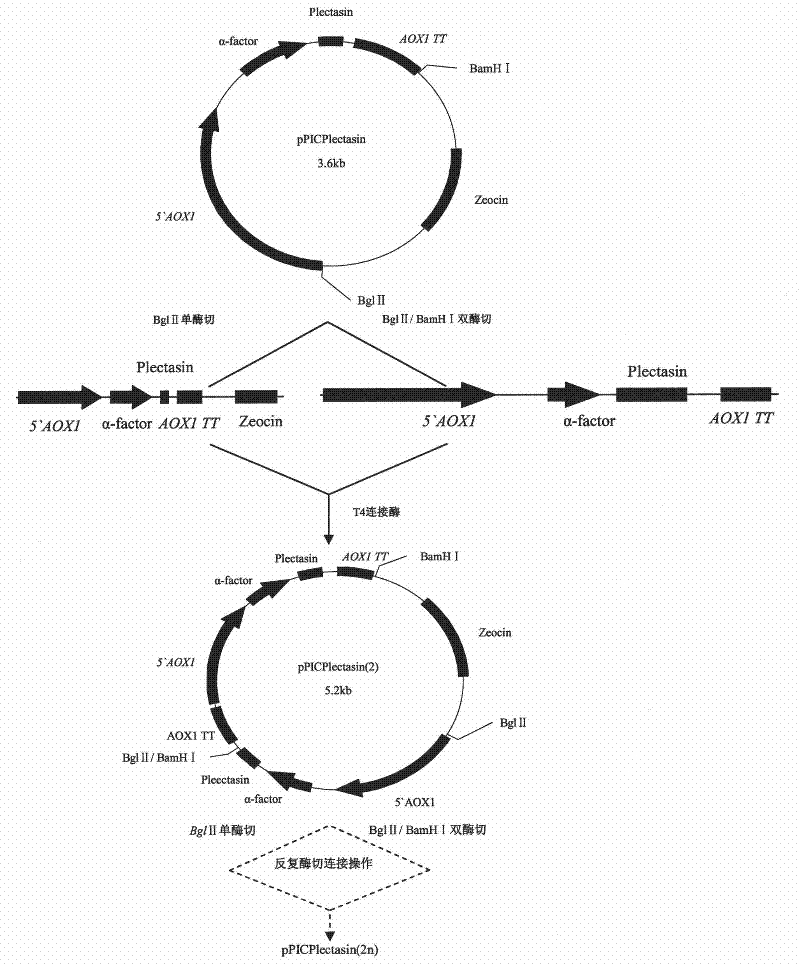
Multi-copy high expressed recombined plectasin by pichia pastoris
ActiveCN102409003AIncrease expression abundanceFungiAntibody mimetics/scaffoldsPichia pastorisPlectasin
The invention discloses a preparation method of multi-copy high expressed recombined plectasin by pichia pastoris. The method comprises the following steps: a plectasin expressing gene sequence is designed according to preference performance to codon translated by pichia pastoris; the optimized plectasin gene is fused on an alpha-factor signal peptide C terminus of an expression vector pPICZalphaA to construct a single-copy expression vector, the vector comprises a plectasin expression cassette containing a start signal element alcohol oxygen dehydrogenase strong promoter (AOX), alpha-factor signal peptide gene and a plectasin gene fused in C terminus, a stop signal element AOX (TT) and the like. A complementation principle of restriction endonuclease Bg1II and BamHI cohesive end is used to obtain plectasin gene-containing recombinant plasmid of different copy cascade expression cassettes, pichia pastoris is electrotransformed and secreted and expressed plectasin with high efficiency under the methanol induction. The expression level and plectasin gene copy number exist a linear relation. The constructed multi-copy high expressed yeast cells can be used for raising the output and reducing the cost, and is adapted to large scale production of plectasin.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Genetically engineered bacterium for producing glucose oxidase as well as construction and application thereof
The invention discloses a genetically engineered bacterium for producing glucose oxidase as well as a construction method and an application thereof, and belongs to the technical field of genetic engineering. A recombinant DNA (Deoxyribonucleic Acid) technology is used for cloning and connecting an Aspergillus niger glucose oxidase (GOD) gene to a Pichia pastoris expression carrier pPIC9K and converting Pichia pastoris GS115; screening and identifying to obtain recombinant Pichia pastoris GS115-pPIC9K-GOD which can produce the glucose oxidase with the higher activity and has the preservation number of CCTCC (China Center For Type Culture Collection) NO: M2012266. The glucose oxidase expressed by the bacterial strain has the enzyme activity of 52 U / mL in a shaking bottle and the enzyme activity is improved by about 24 times as compared with the enzyme activity of wild funguses, so as to lay a good foundation for the large-scale production of the glucose oxidase.
Owner:JIANGNAN UNIV
Preparation and application of recombinant plectasin
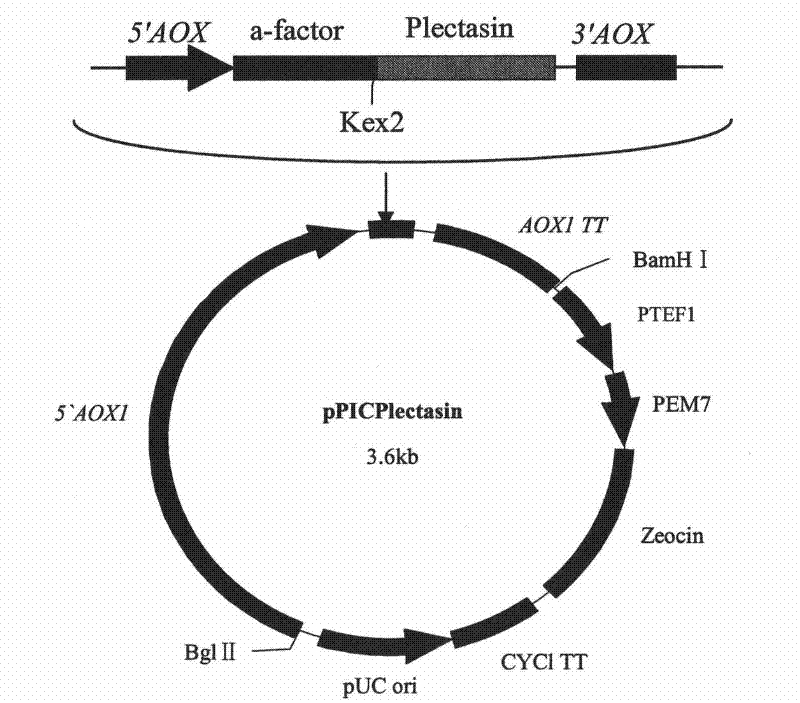
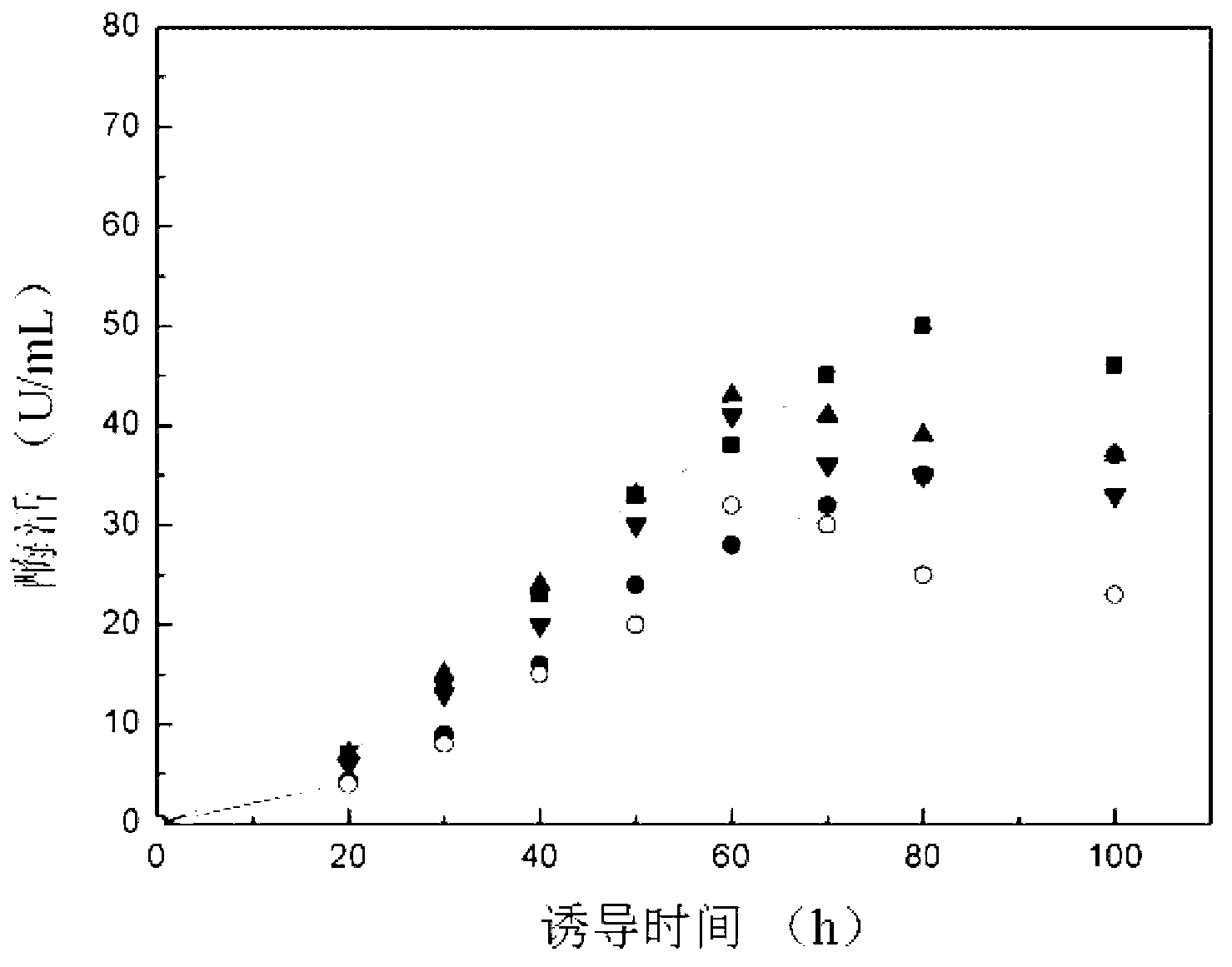
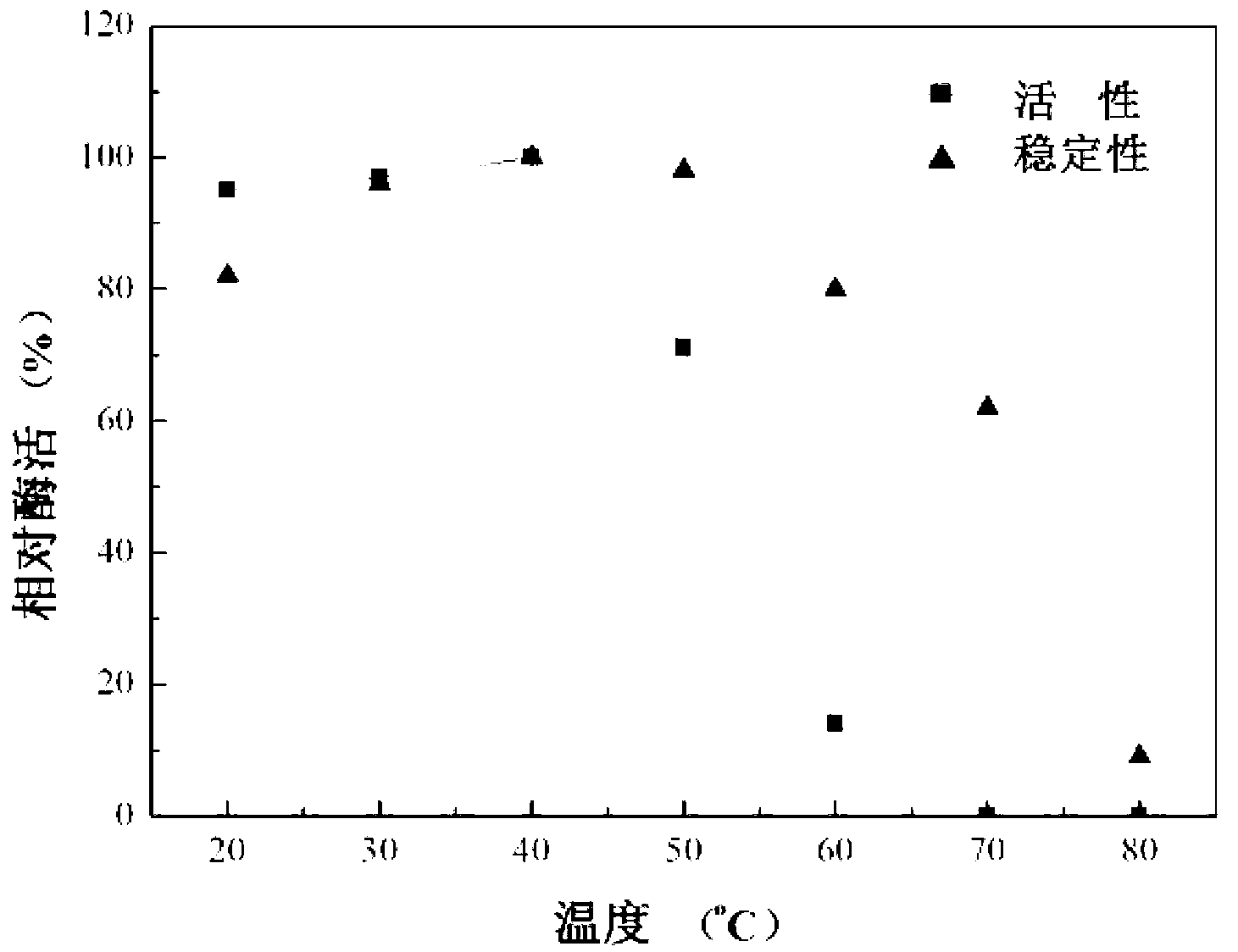
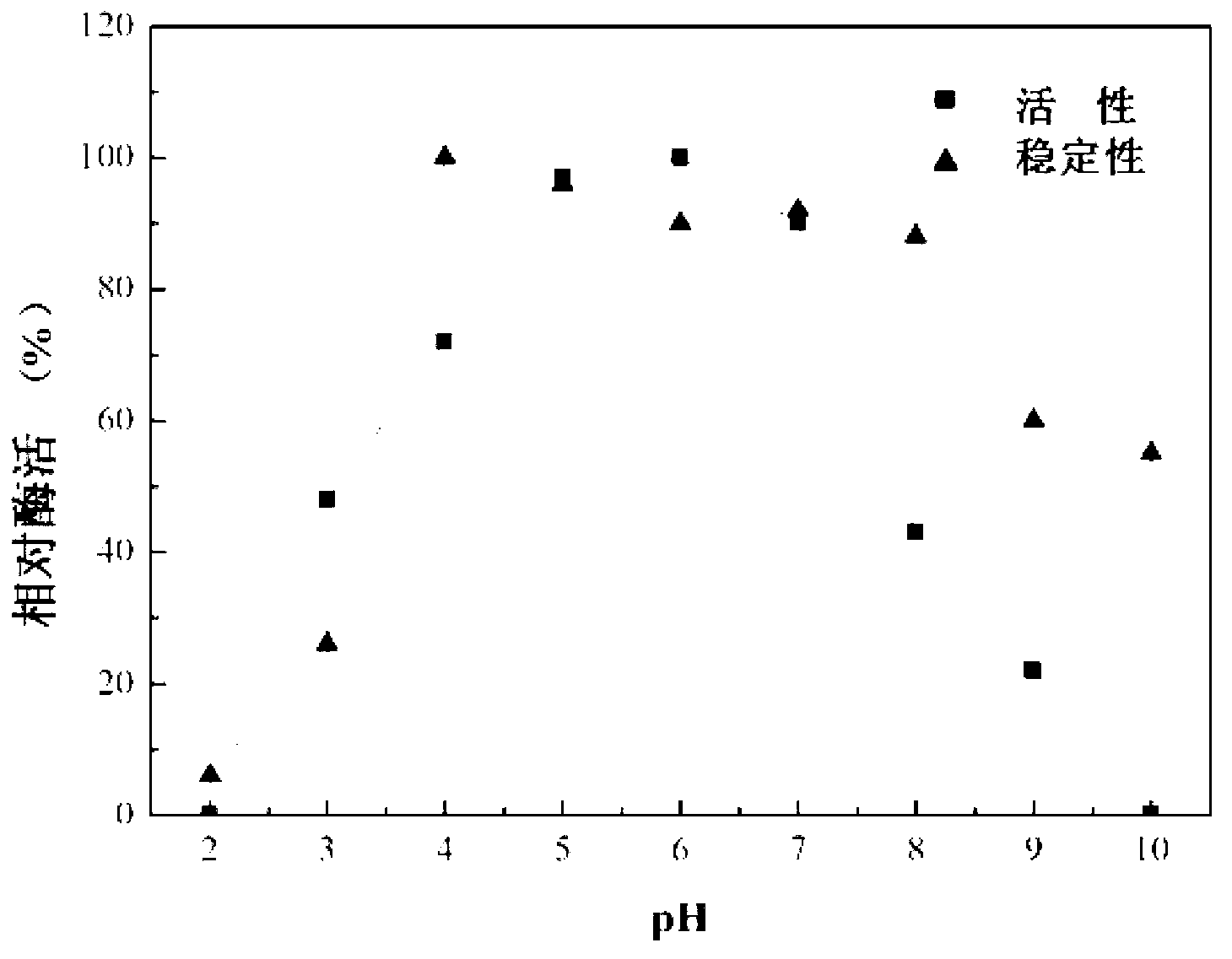
The invention discloses preparation and application of recombinant plectasin. The method comprises designing plectasin gene according to preferred codons of Pichia pastoris, wherein possible nucleotide sequences of the plectasin gene are expressed in SEQ ID NO. 1, constructing recombinant expression vectors pPICPlectasin and recombinant genetic engineering bacteria Pichia pastoris X33pPICPlectasin (CGMCC NO. 3564), carrying out a high density fermentation process on the recombinant genetic engineering bacteria Pichia pastoris having a high expression level, wherein a total protein concentration of supernate from the high density fermentation process is 729 microgrammes per milliliter, dialyzing and freeze-drying the supernate, and orderly carrying out a gel filtration chromatography treatment and a reversed phase high performance liquid chromatogram treatment on the freeze-dried supernate to obtain high purity recombinant plectasin. The high purity recombinant plectasin is not hemolytic, has favorable PH stability, heat stability and anti-pepsin activity, and can inhibit effectively the growth of gram-positive pathogen Streptococcus pneumonia, staphylococcus aureus and staphylococcus epidermidis. Therefore, the high purity recombinant plectasin can be utilized for treating and preventing gram-positive bacterium and especially streptococcus and has potential antimicrobial drug development values.
Owner:SHENZHEN SUNSMILE BIOTECH
Optimized beta-mannase gene MAN and pichia pastoris expression vector thereof
ActiveCN103898133AImprove expression levelGreat application potentialFungiMicroorganism based processesPichia pastorisYeast
The invention relates to the field of genetic engineering and specifically relates to an optimized beta-mannase gene MAN and a pichia pastoris expression vector thereof. A beta-mannase gene with a nucleotide sequence as shown in SEQ ID NO.1 is modified to obtain an optimized gene of which the nucleotide sequence is as shown in SEQ ID NO.3. A recombinant pichia pastoris expression vector of the optimized beta-mannase gene is constructed; and the expression vector is transferred in a pichia pastoris competent cell X33 to obtain a trans-beta-mannase pichia pastoris bacterial strain capable of efficiently expressing beta-mannase by screening; an enzymatic property test indicates that the optimized beta-mannase gene can be stably and efficiently expressed and inherited in the pichia pastoris; enzyme activity and stability of the expressed beta-mannase are remarkably higher than those of the beta-mannase expressed in the original beta-mannase gene pichia pastoris.
Owner:GUANGDONG VTR BIO TECH
Pichia pastoris for producing methanol protein and lipase at same time and application thereof
The invention relates to a pichia pastoris for producing methanol protein and lipase at the same time and application thereof. The problems that during existing production of methanol protein, products are single, the fermentation technology is old, cost is high, and benefits are low are effectively solved. The pichia pastoris for producing methanol protein and lipase at the same time is named as pichia pastoris zfwx-208 strain and preserved in the China center for type culture collection, the preservation number is CCTCC NO.M2013518, the preservation date is October 30th, 2013, and the preservation address is the China center for type culture collection in Wuhan university of Wuhan city in China. Because of combined fermenting production of lipase and methanol protein, a device is multipurpose, production cost is reduced, production benefits are improved, the production technology is simplified, raw material consumption is reduced, energy in use is saved, and environmental pollution is relieved.
Owner:义马煤业集团煤生化高科技工程有限公司
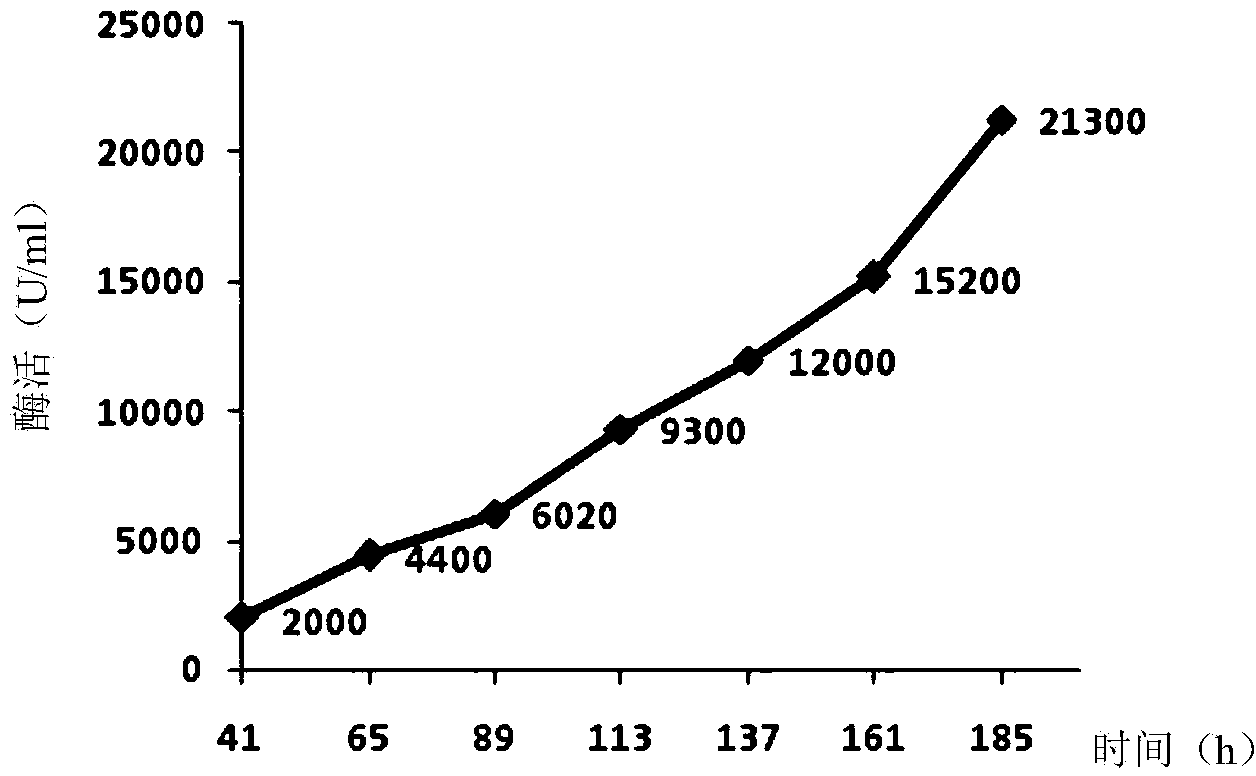
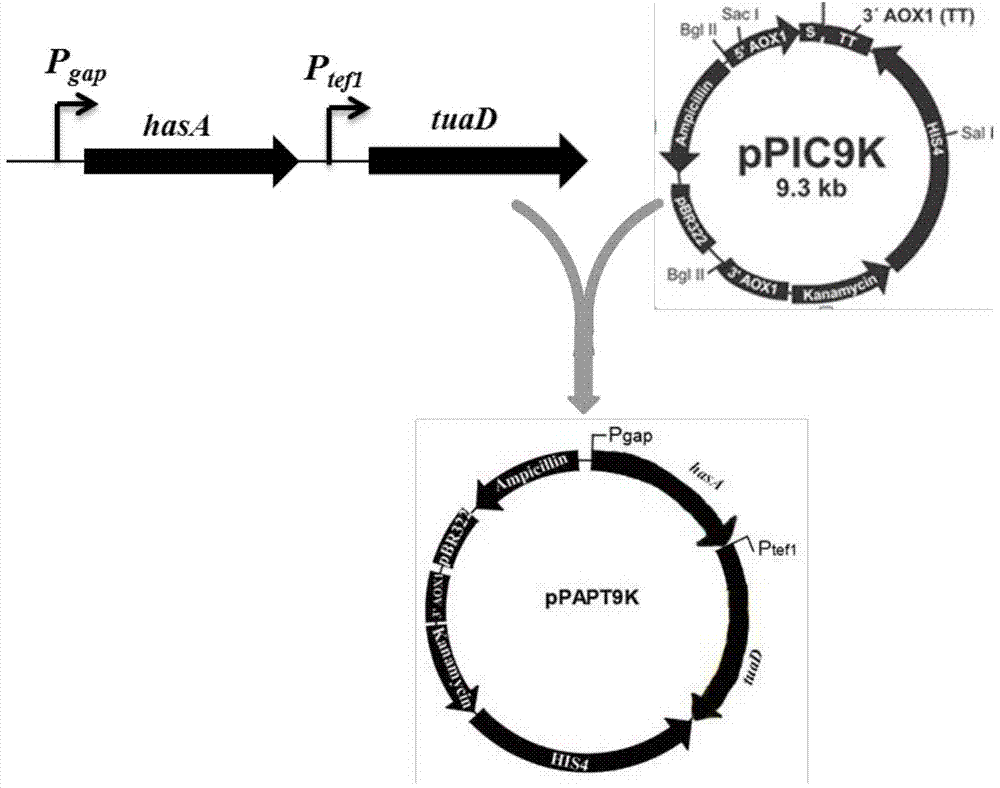
Recombinant Pichia pastoris for producing small-molecular hyaluronic acids and construction method thereof
ActiveCN104263666AImprove conversion yieldIncrease productionFungiMicroorganism based processesBiotechnologyStreptococcus zooepidemicus
The invention discloses a recombinant Pichia pastoris for producing small-molecular hyaluronic acids and a construction method thereof, belonging to the technical field of bioengineering. Hyaluronic acid synthase hasA derived from Streptococcus zooepidemicus and UDP-glucose dehydrogenase tuaD derived from Bacillus subtilis are expressed in recombinant Pichia pastoris GS115 host to implement production of hyaluronic acids. Meanwhile, hyaluronidase derived from hirudo is integrated to the Pichia pastoris genome and subjected to secretion expression by constitutive promoters with different intensities; the secretion expression level of the hyaluronidase is controlled to prepare small-molecular hyaluronic acid products with different molecular weights; and the prepared products have different ranges of molecular weight, and have instruction and reference meanings for directly producing small-molecular hyaluronic acids within a specified range from microbes. The recombinant Pichia pastoris lays certain foundation for high-efficiency preparation of small-molecular hyaluronic acids, and is suitable for industrial production.
Owner:JIANGNAN UNIV
Pichia kluyveri var.kluyveri strain, culture method and application thereof
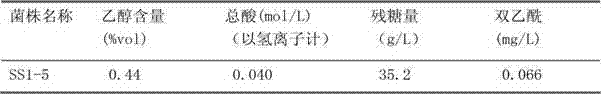
The invention relates to a pichia kluyveri var.kluyveri strain, a culture method of the pichia kluyveri var.kluyveri strain and an application of the pichia kluyveri var.kluyveri strain; the pichia kluyveri var.kluyveri strain SS1-5 has been preserved in China General Microbiological Culture Collection Center (CGMCC) since December 20, 2010, wherein the address of the CGMCC is Institute of Microbiology, Chinese Academy of Sciences, 3#, First Yard, West Beichen Road, Chaoyang District, Beijing, and the preservation number (CGMCC No.) of the strain is 4494. The strain can be used for brewing low-alcohol beverages that are ample in fruit, low in alcohol content, sweet in mouth feel and closer to alcohol-free beer in flavor and nutrition; meanwhile, the low-alcohol beverages can keep organic nutrition in malt wort in a better way; and therefore, the production technology is simplified, the cost and the energy consumption are reduced and the environmental pollution is alleviated.
Owner:QILU UNIV OF TECH
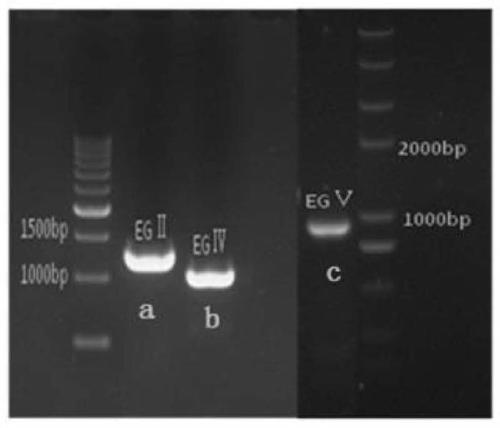
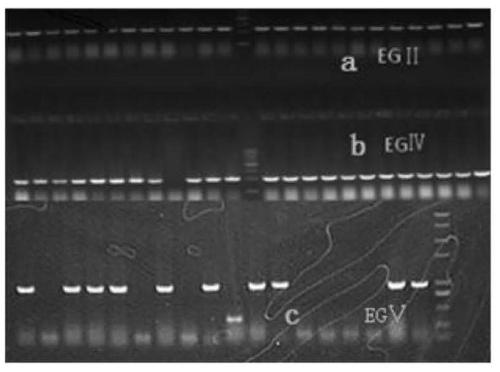
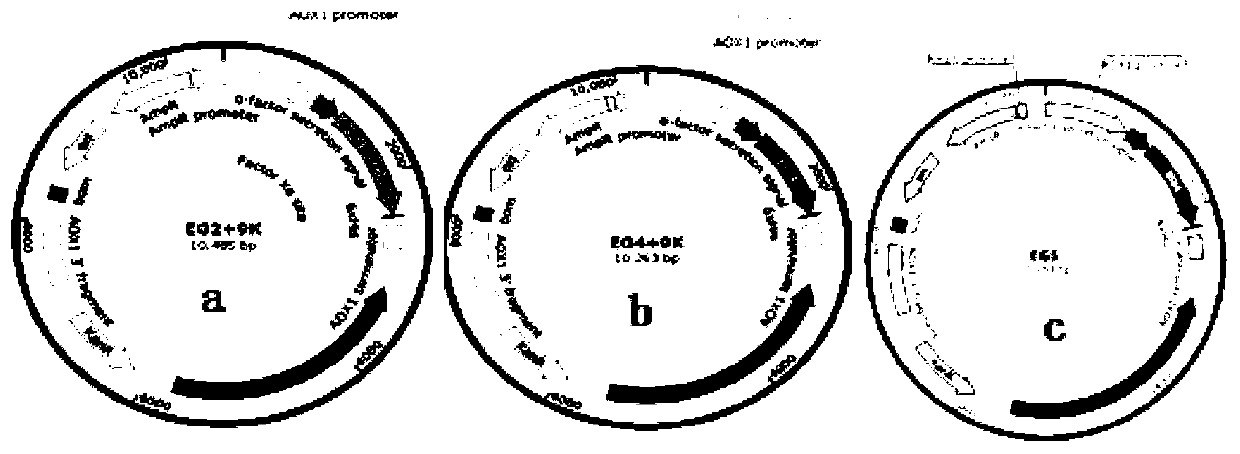
Method for constructing heterologous expression endoglucanases EG II, EG IV and EG V in pichia pastoris
InactiveCN109971784AOptimized primer sequencesEasy to purifyGlycosylasesVector-based foreign material introductionHeterologousPichia pastoris
The invention aims to construct recombinant pichia pastoris for heterologous expression of EG II, EG IV and EG V proteins. A method comprises the following specific steps: (1) obtaining of trichodermareesei RNA and CDNA: obtaining mycelia by using a solid induction culture medium, and extracting total RNA and cDNA by using a kit; (2) cloning of a target gene: amplifying a target gene by a reasonable primer PCR; (3) constructing an expression vector: taking Ppic9k as a vector, inserting the target gene into the downstream of a promoter AOX1, wherein the 5 ' and 3 ' end enzyme digestion sites are EcoR I and Not I respectively; (4) obtaining of a recombinant strain: taking bgl I as a linearization site of an expression vector to realize linearization and electrotransformation of the expression vector to obtain recombinant yeast; (5) induction and enzyme production of the recombinant strain: carrying out shaking flask fermentation, and inducing enzyme production by using appropriate methanol; and (6) recombinant protein activity detection: detecting the expression and activity of recombinant protein by SDS-PAGE and Congo red-CMC. The recombinant strain constructed by the method can efficiently express the protein with a his label, and a high-efficiency single enzyme component can be obtained.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
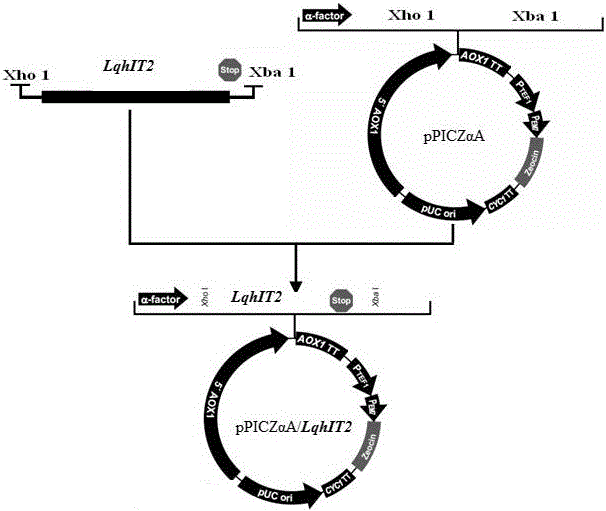
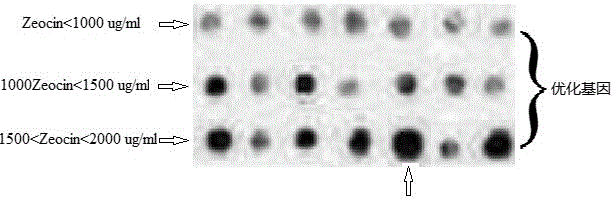
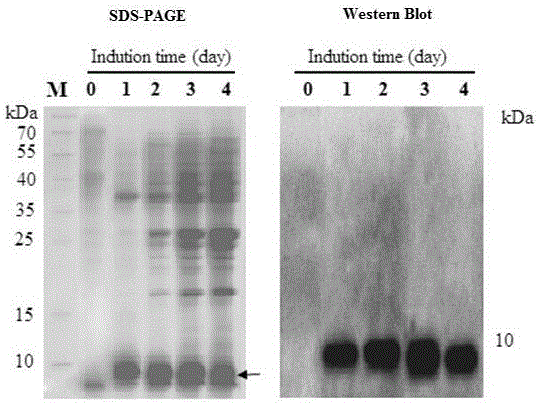
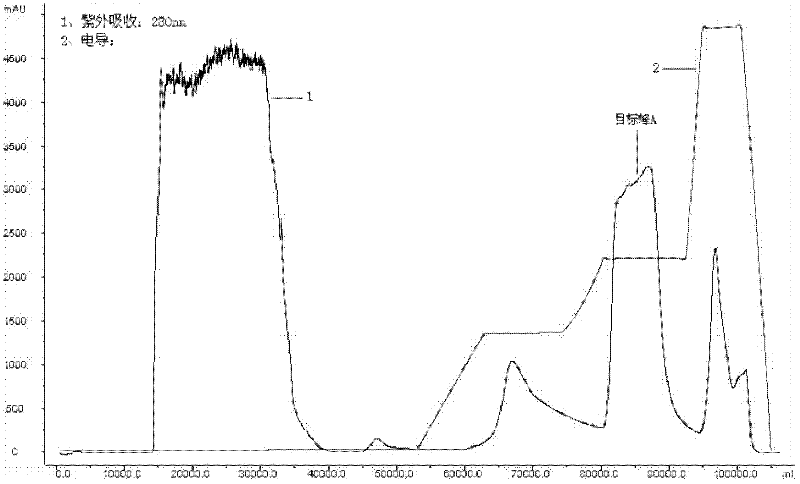
Method for preparing recombinant scorpion neurotoxin LqhIT2 protein
ActiveCN106701774AToxicLow toxicityMicroorganism based processesPeptide preparation methodsEscherichia coliPichia pastoris
The invention discloses a method for efficiently preparing recombinant active scorpion insect neurotoxin LqhIT2. The method mainly comprises a step (1) of optimizing and synthesizing LqhIT2 genes with C- ends provided with 6*His labels according to amino acid sequences of mature LqhIT2 toxins and characteristics of a pichia pastoris expression system and constructing pichia pastoris secretory expression vectors; a step (2) of converting recombinant vectors into a pichia pastoris host, and through screening, obtaining yeast transformants in high-level secretory expression; a step (3) of expressing recombinant scorpion insect neurotoxin LqhIT2 proteins in the pichia pastoris host, and quickly obtaining the recombinant scorpion insect neurotoxin LqhIT2 proteins with the purity over 98% through a Ni2+ affinity chromatography method, wherein the purified recombinant proteins have very strong poisonous activity on insect cells. The method is modified based on conventional expression of insect neurotoxin LqhIT2 through prokaryotic host escherichia coli, the method of using eukaryotic host pichia pastoris to express the insect neurotoxin LqhIT2 is explored, and the yeast transformants in high-level secretory expression are screened.
Owner:HUAIHUA UNIV
Production method for Pichia pastoris expression recombinant human interleukin 11
ActiveCN102329388AReduce preprocessingConvenient purification workPeptide preparation methodsInterleukinsPichia pastorisInterleukin 11
The invention discloses a production method for Pichia pastoris expression recombinant human interleukin 11, which comprises the step of combined purification sequentially through reverse phase column chromatography, hydrophobic column chromatography and gel column chromatography. By adopting the production method, high-purity medicinal recombinant human interleukin 11 proteins can be prepared ina large scale.
Owner:HANGZHOU JIUYUAN GENE ENG
Lipase, engineering bacterium and preparing methods of the lipase and the engineering bacterium
InactiveCN104480083AIncrease heterologous expressionHigh expressionFungiHydrolasesPichia pastorisHeterologous
A lipase, an engineering bacterium and preparing methods of the lipase and the engineering bacterium are disclosed. The gene sequence of the lipase is a gene sequence shown as SEQ ID No.1 or an isogenous gene sequence with same functions. The host bacterium of the engineering bacterium is pichia pastoris. The gene sequence of the lipase is inserted at an His locus. The preparing method of the engineering bacterium includes: (1) constructing a lipase genetic expression vector; (2) constructing a molecular chaperone composite genetic expression vector; (3) preparing a lipase-expressed transgenic engineering bacterium; and (4) preparing the high-lipase-expression engineering bacterium. Preparation of the lipase by utilization of the engineering bacterium includes steps of: A) activating to obtain a seed solution; B) fermenting in a fermentation tank; and C) centrifuging and collecting supernate liquid. The heterologous expression of the lipase in the engineering bacterium is increased. The engineering bacterium has a secretion property, and is high in unit enzyme activity, convenient in production and good in economic benefit.
Owner:HUAZHONG UNIV OF SCI & TECH RES INST SHENZHEN +1
Recombinant pichia pastoris for heterogenous efficient expression of lipase and application of recombinant pichia pastoris
ActiveCN107083373AImprove secretion efficiencyHigh expressionFungiHydrolasesPichia pastorisHeterologous
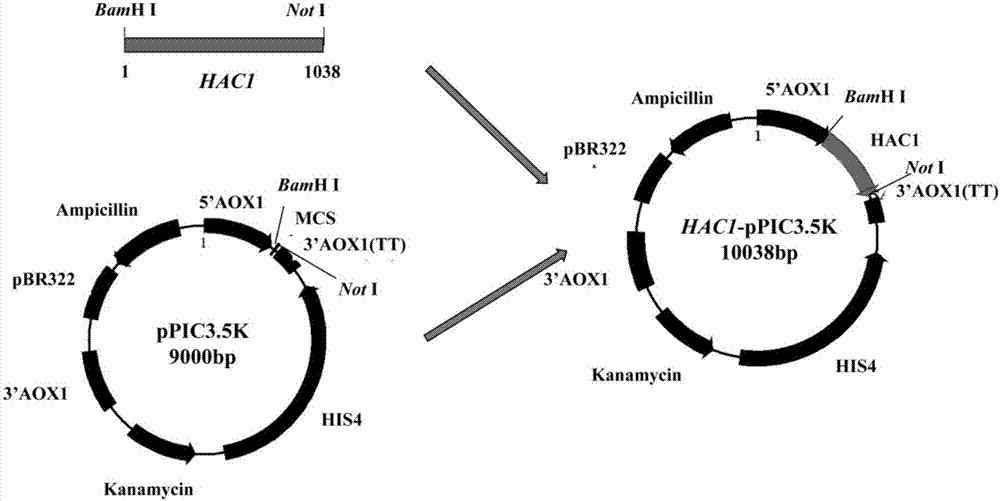
The invention discloses recombinant pichia pastoris for heterogenous efficient expression of lipase and application of the recombinant pichia pastoris. The recombinant pichia pastoris is obtained by converting plasmid HAC1-pPIC3.5K of an overexpression HAC1 gene in recombinant pichia pastoris X-33 which contains a pro-rml gene of a copy number 4 and is capable of expressing Pro-RML. When the strain is subjected to 96 hours of flask shaking fermentation, the extracellular enzyme activity is up to 1078U / mL at most, and the enzyme activity secretion efficiency is up to 47U / OD600. 120 hours of fermentation is needed when pichia pastoris which is disclosed by patent CN103361327A and has a rhizomucor mieheilipase copy number of 2 meets the highest extracellular enzyme activity and enzyme secretion efficiency, the extracellular enzyme activity is 1038U / mL at most, and the enzyme activity secretion efficiency is only 25U / OD600. By adopting the recombinant pichia pastoris, expression of rhizomucor mieheilipase is effectively promoted, on the premise that the highest extracellular enzyme activity is not influenced, the secretion efficiency of the rhizomucor mieheilipase is increased by 1.9 times, and the fermentation time is shortened by 24 hours.
Owner:XUZHOU NORMAL UNIVERSITY
Promoter increasing expression level of foreign proteins of pichia pastoris and application of promoter
ActiveCN109735547AHigh expressionReduce manufacturing costHydrolasesMicroorganism based processesBiotechnologyPichia pastoris
The invention provides a novel promoter. The promoter can significantly increase the expression level of foreign proteins of pichia pastoris. The promoter can increase the expression level of enzyme genes such as hemicellulase, peroxidase and protease in the pichia pastoris by 76-320%, and the effect is remarkable, thereby facilitating the great reduction of the production cost of the enzymes andpromoting the wide application of the enzymes.
Owner:QINGDAO VLAND BIOTECH GRP
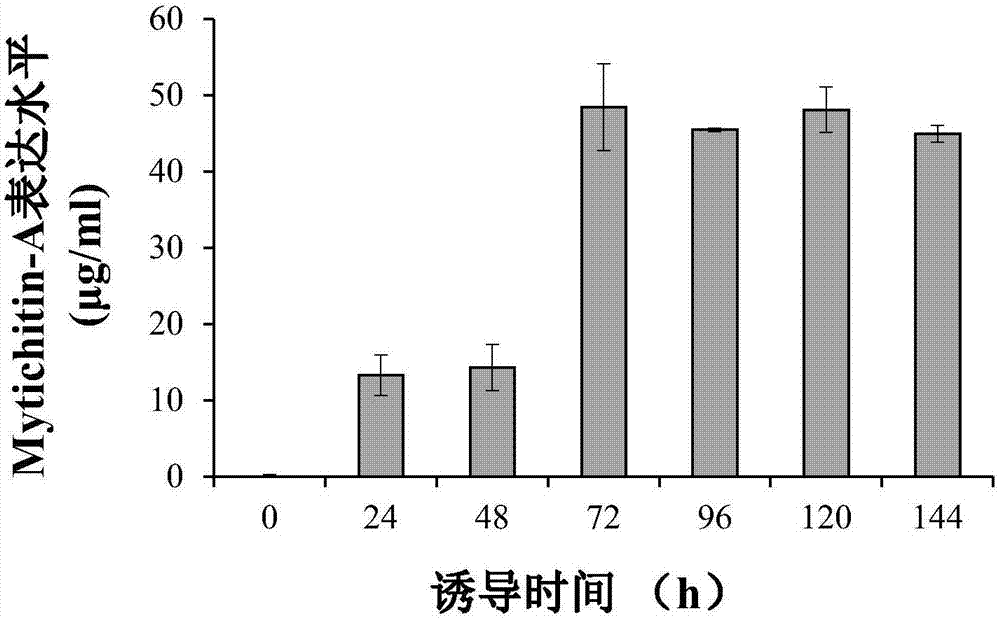
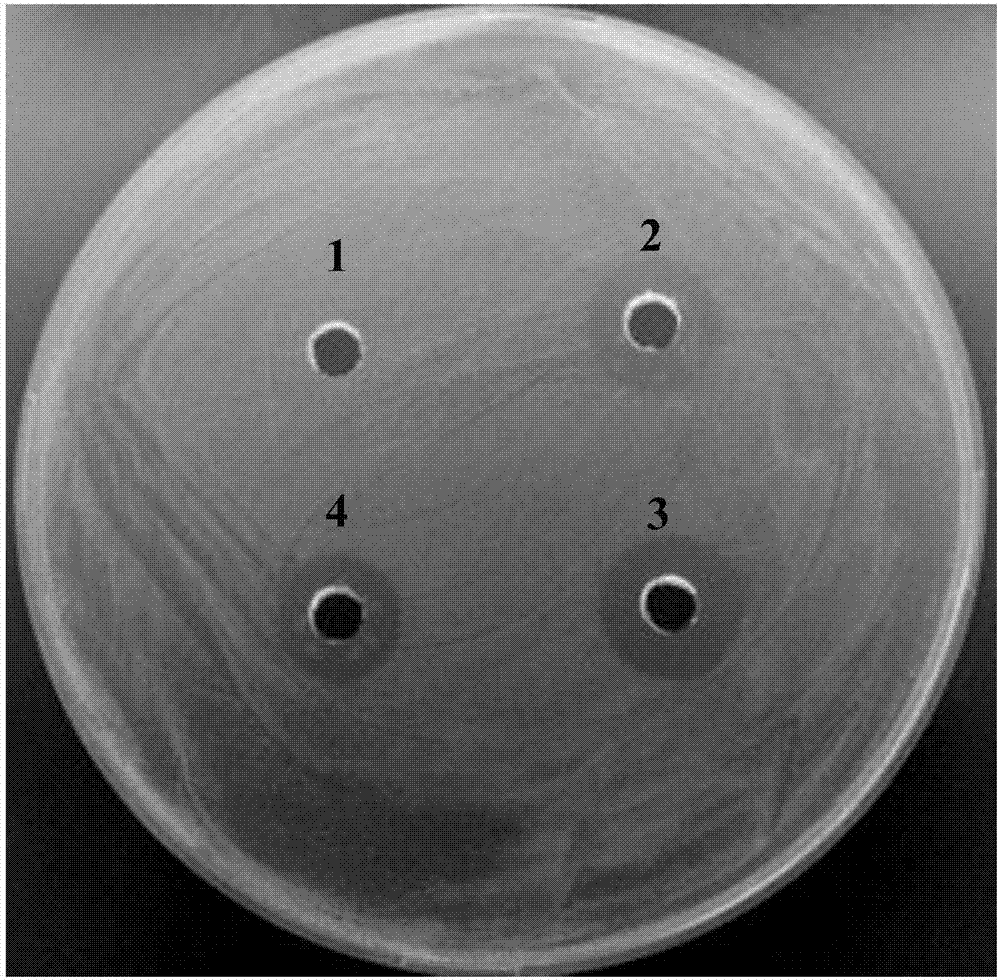
High efficient preparation of novel antimicrobial peptide Mytichitin-A in pichia pastoris
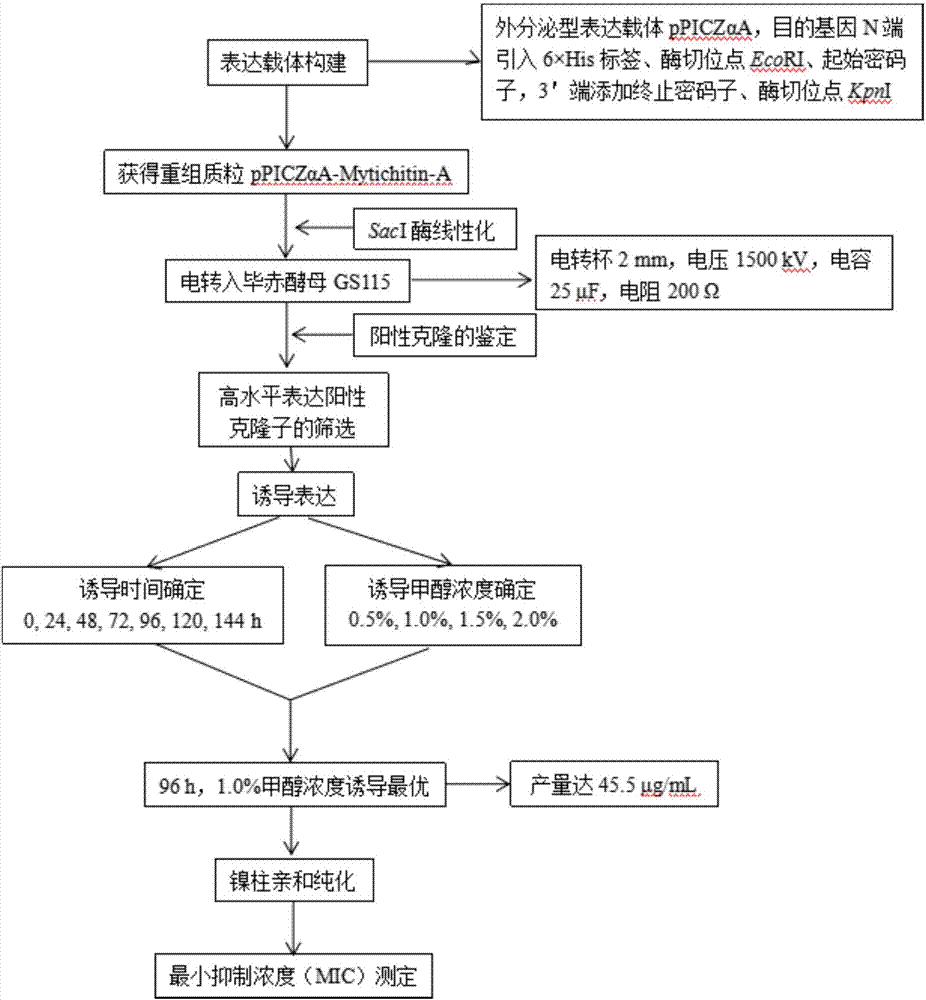
The invention discloses a method for high efficient preparation of novel Mytilus coruscus antimicrobial peptide Mytichitin-A in pichia pastoris. A gene engineering method is adopted and the full-length gene of the antimicrobial peptide Mytichitin-A is inserted into an exosome type expression vector pPICZalphaA through EcoRI and KpnI restriction enzyme cutting sites, so as to obtain a pPICZalphaA-Mytichitin-A recombinant expression vector. Through an electrotransformation method, a SacI enzyme linearized recombinant plasmid pPICZalphaA-Mytichitin-A is transferred into the pichia pastoris GS115. Through optimizing, the optimum expression conditions are determined as follows: methanol concentration is 1.0%, fermentation time is 96h, and the yield is 45.5 microgram / mL. The result of bacteriostasis experiments shows that the recombinant antibacterial peptide has an obvious inhibition effect on both staphylococcus aureus and bacillus subtilis 151-1.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Aspergillus nidulans chitin deacetylase, and preparation method and application thereof
InactiveCN109022403AHigh deacetylation activityImprove biological activityHydrolasesFermentationBiotechnologyPichia pastoris
The invention discloses an Aspergillus nidulans chitin deacetylase, and a preparation method and an application thereof. The sequence of a chitin deacetylase coding gene in Aspergillus nidulans is obtained by a whole gene synthesis technology according to the codon preference of Pichia yeast, and the optimized nucleic acid sequence is represented by SEQ ID NO.2. The optimized chitin deacetylase encoding gene is secreted and expressed by a Pichia pastoris expression system to obtain the Aspergillus nidulans chitin deacetylase, and the amino acid sequence of the Aspergillus nidulans chitin deacetylase is represented by SEQ ID NO.1. The Aspergillus nidulans chitin deacetylase obtained in the invention can remove acetyl groups in chitosan and chitosan oligosaccharides in order to obtain the chitosan or chitosan oligosaccharides with a specific structure. The modified chitosan or chitosan oligosaccharides have new or higher biological activities than unmodified chitosan or chitosan oligosaccharides. So the above enzyme and its deacelation product have good industrial application prospects.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Novel lipase gene, lipase production strain and application
ActiveCN102965384AWith medium and high temperature resistanceAlkaline resistantFungiBacteriaYeastPichia pastoris
Belonging to the field of enzymatic deinking, the invention discloses a novel lipase gene, a lipase production strain and application. The novel lipase gene is obtained by codon optimization, and can realize high expression in Pichia Pastoris. By connecting the gene to a Pichia Pastoris expression plasmid pPICZ alpha, a plasmid pPICZ alpha A-ARL can be obtained. Then the plasmid pPICZ alpha A-ARL is employed to convert a Pichia Pastoris host bacterium Pichia Pastoris X33, thus obtaining the lipase production strain Pichia Pastoris X33 / pPICZ alpha A-ARL. The strain can achieve high-efficiency lipase expression. The expressed lipase has an activity of 2500U / mL, and has medium and high temperature resistance as well as alkali resistance, thus being applicable to old newspaper deinking effectively.
Owner:SOUTH CHINA UNIV OF TECH +1
Construction and application of ganoderma laccase pichia pastoris genetic engineering strain
InactiveCN105349558AHigh copy numberHigh activityFungiMicroorganism based processesBiotechnologyPichia pastoris
Owner:SHANGHAI JIAO TONG UNIV
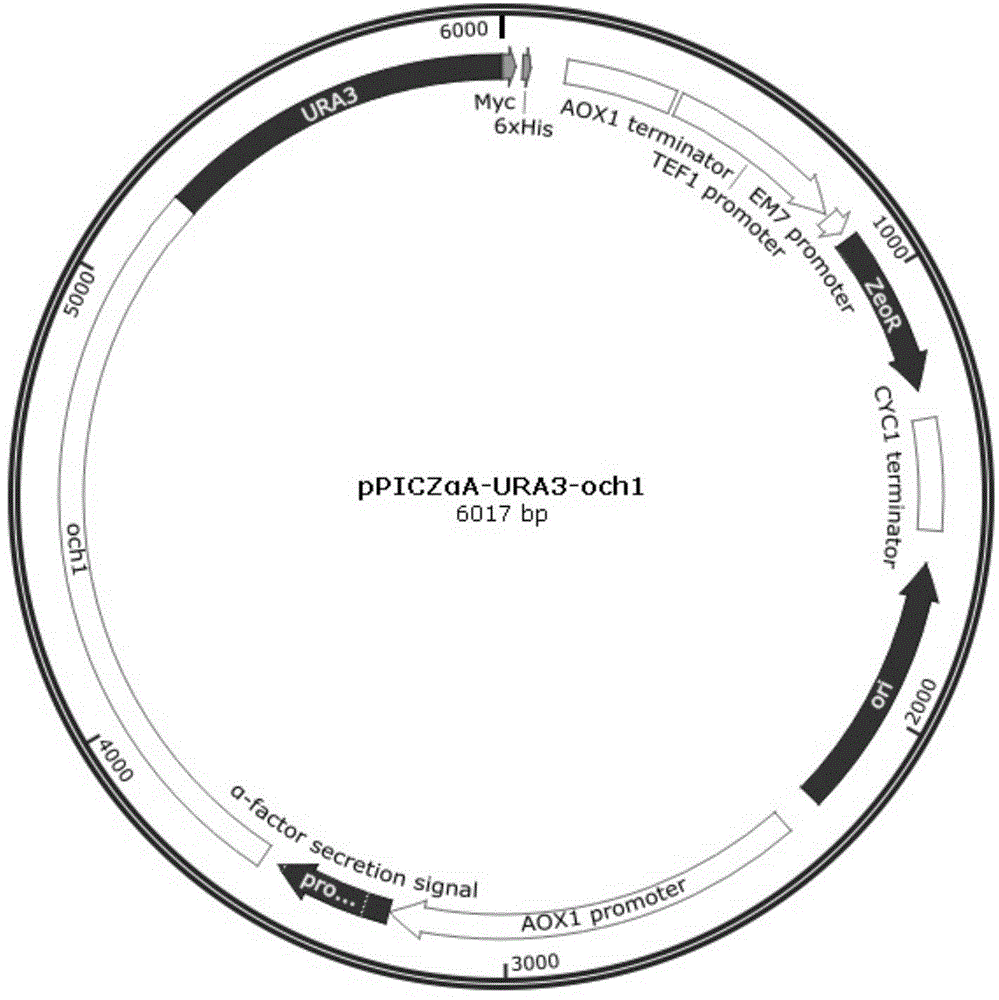
Pichia pastoris strains for producing predominantly homogeneous glycan structure
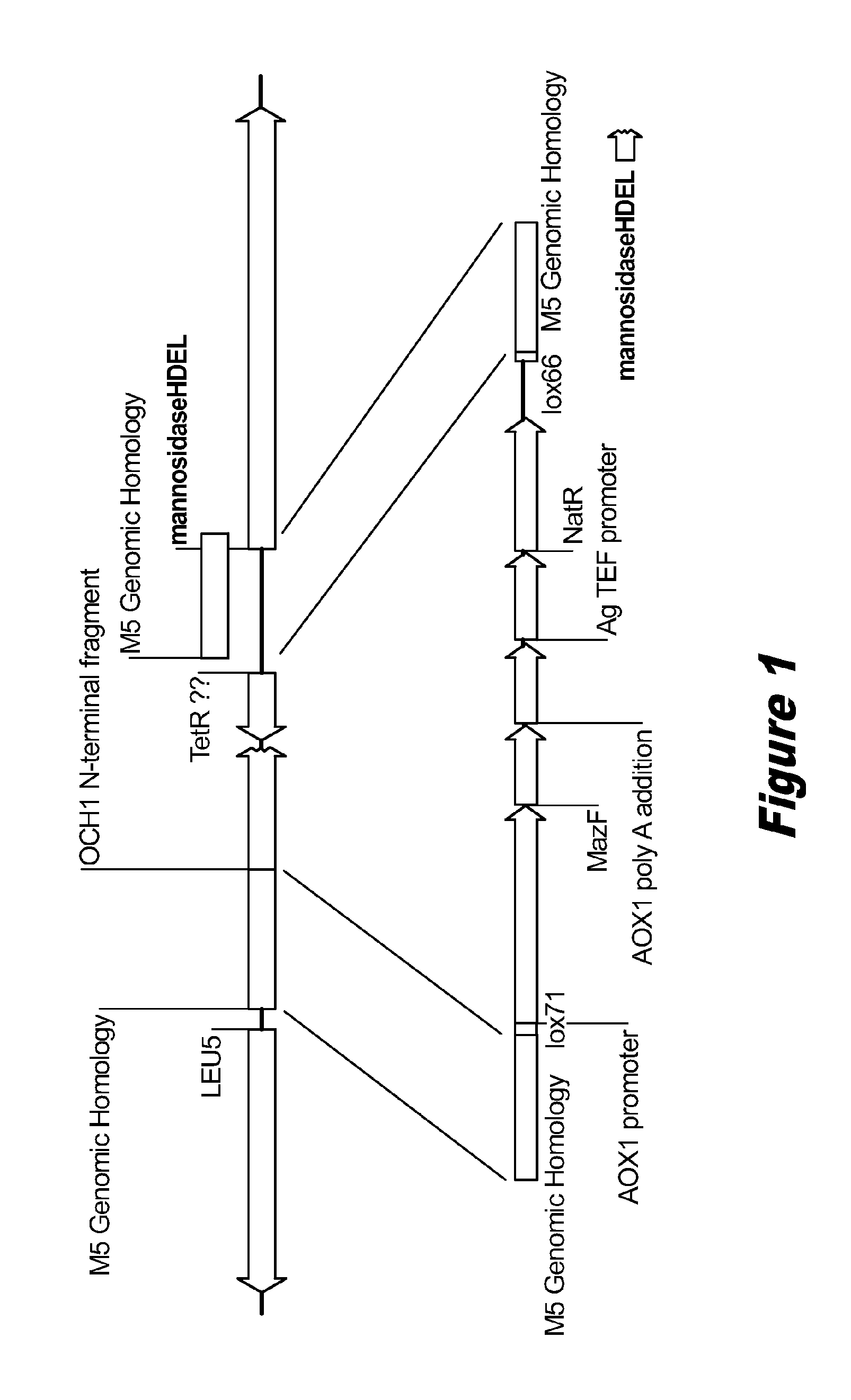
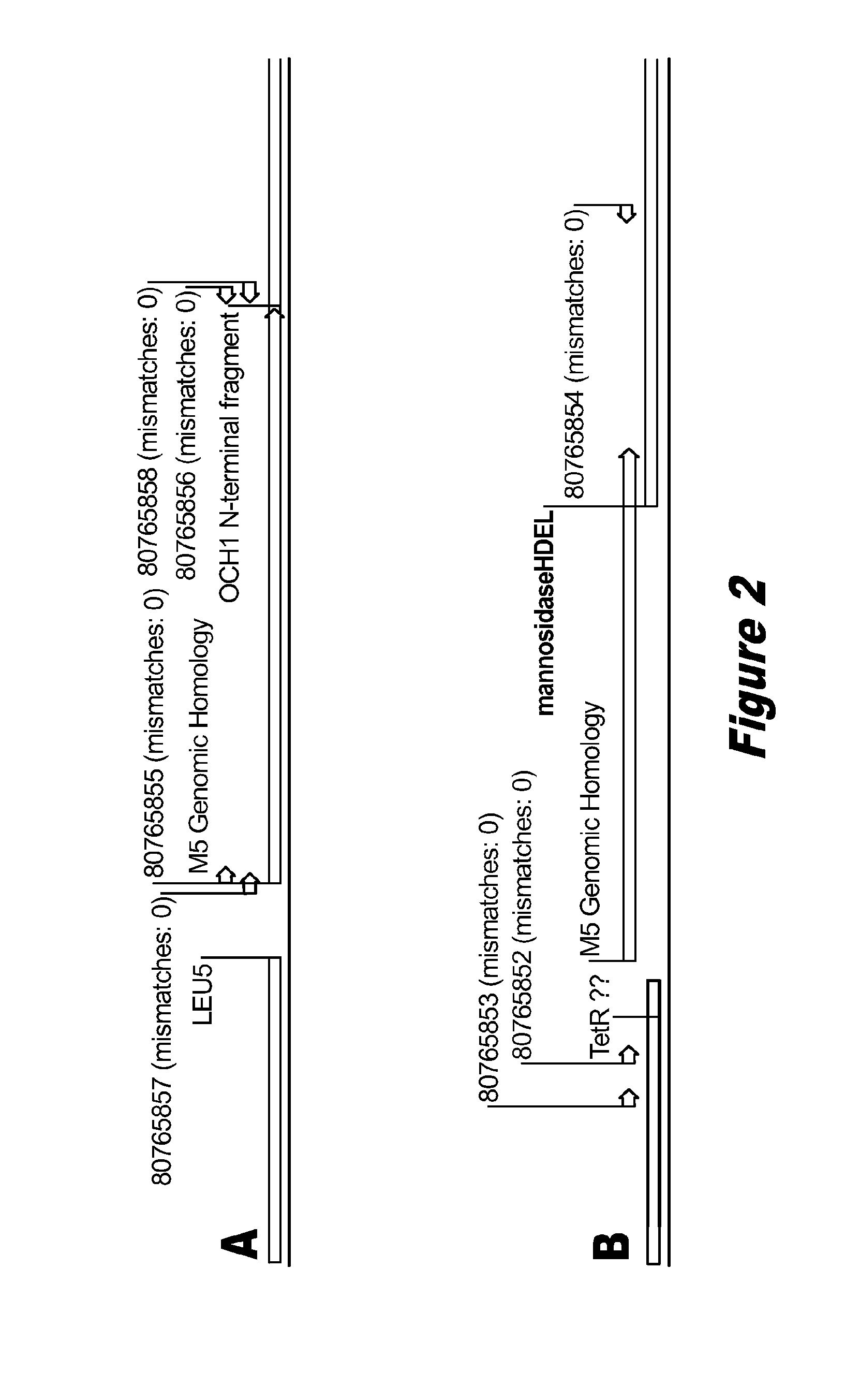
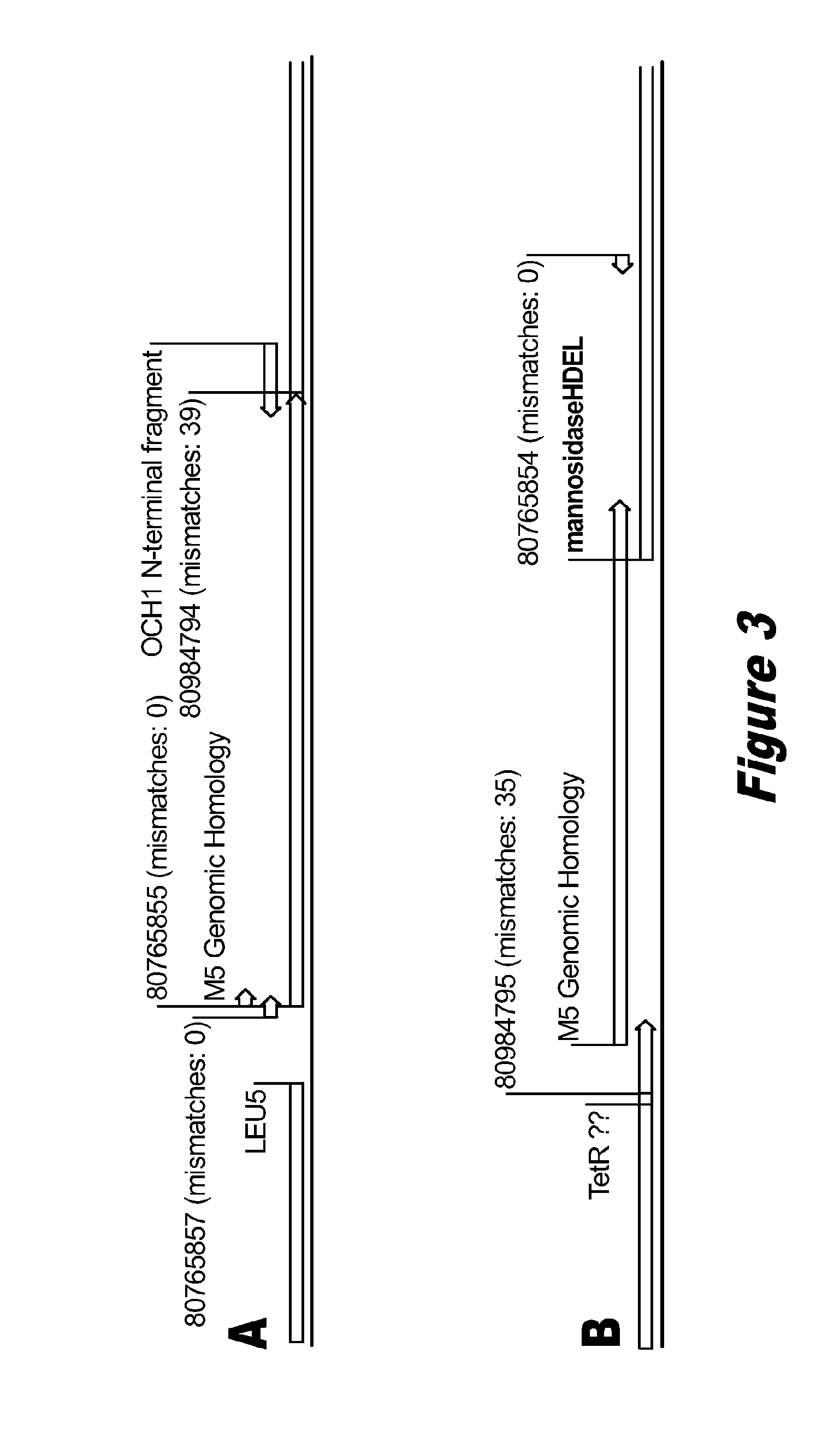
Disclosed herein are novel Pichia pastoris strains for expression of exogenous proteins with substantially homogeneous N-glycans. The strains are genetically engineered to include a mutant OCH1 allele which is transcribed into an mRNA coding for a mutant OCH1 gene product (i.e., α-1,6-mannosyltransferase, or “OCH1 protein”). The mutant OCH1 protein contains a catalytic domain substantially identical to that of the wild type OCH1 protein, but lacks an N-terminal sequence necessary to target the OCH1 protein to the Golgi apparatus. The strains disclosed herein are robust, stable, and transformable, and the mutant OCH1 allele and the ability to produce substantially homogeneous N-glycans are maintained for generations after rounds of freezing and thawing and after subsequent transformations.
Owner:RES CORP TECH INC
Anthropogenic lysozyme encoding gene and expressing method and application thereof in pichia pastoris
ActiveCN109628431AProgressiveAchieve high expressionFungiMicroorganism based processesPichia pastorisYeast
The invention relates to an anthropogenic lysozyme encoding gene and an expressing method and application thereof in pichia pastoris. The anthropogenic lysozyme encoding gene has the nucleotide sequence shown in SEQ ID NO2. Anthropogenic lysozyme regards the eucaryon (pichia pastoris GS115) as the expression host, and in combination with the gene copying number and the cofactor co-expression strategy, the obtained lysozyme product has the advantages of being high in expression amount, high in activity and the like, can serve as an antibiotic substitute and is widely applied in the fields of feed and the like.
Owner:HUBEI UNIV
Cell surface exhibition PET decomposing enzyme reorganizable pichia pastoris and structure and application
ActiveCN106497964ALimited ability to produce enzymesIncrease production costFungiHydrolasesPichia pastorisYeast
The invention discloses a cell surface exhibition PET decomposing enzyme reorganizable pichia pastoris and structure and application, comprising the structured steps of 1, chemo synthesizing the nucleotide sequence of ameliorated PET decomposing enzyme as shown in SEQ ID No. 1; cloning the nucleotide sequence of anchoring protein gene from the gene group of GS115 pichia pastoris; 2, utilizing the OverlapPCR to connect the nucleotide sequence of anchoring protein gene and the nucleotide sequence of ameliorated PET decomposing enzyme and obtaining an amalgamation sequence; 3, connecting the amalgamation sequence to an expression load of pichia pastoris, and obtaining a reorganizable expression load; 4, transferring the reorganizable expression load in a host pichia pastoris, and obtaining a reorganizable pichia pastoris of a cell surface exhibition PET decomposing enzyme. The reorganizable pichia pastoris can anchor the PET decomposing enzyme to the cell surface, the enzyme after Immobilization can be used for an entire cell catalyst, compared to the wild grow type, has high stability, is convenient to recover, easy to control and can be repeatedly utilized.
Owner:TIANJIN UNIV
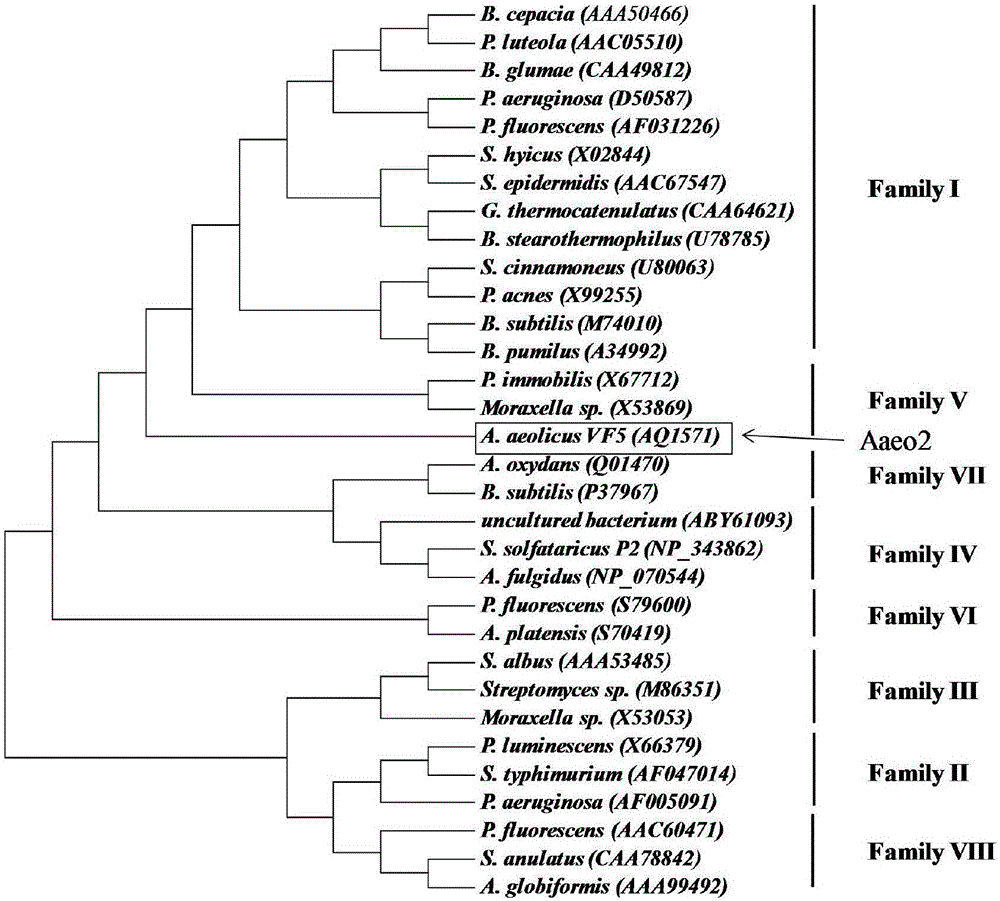
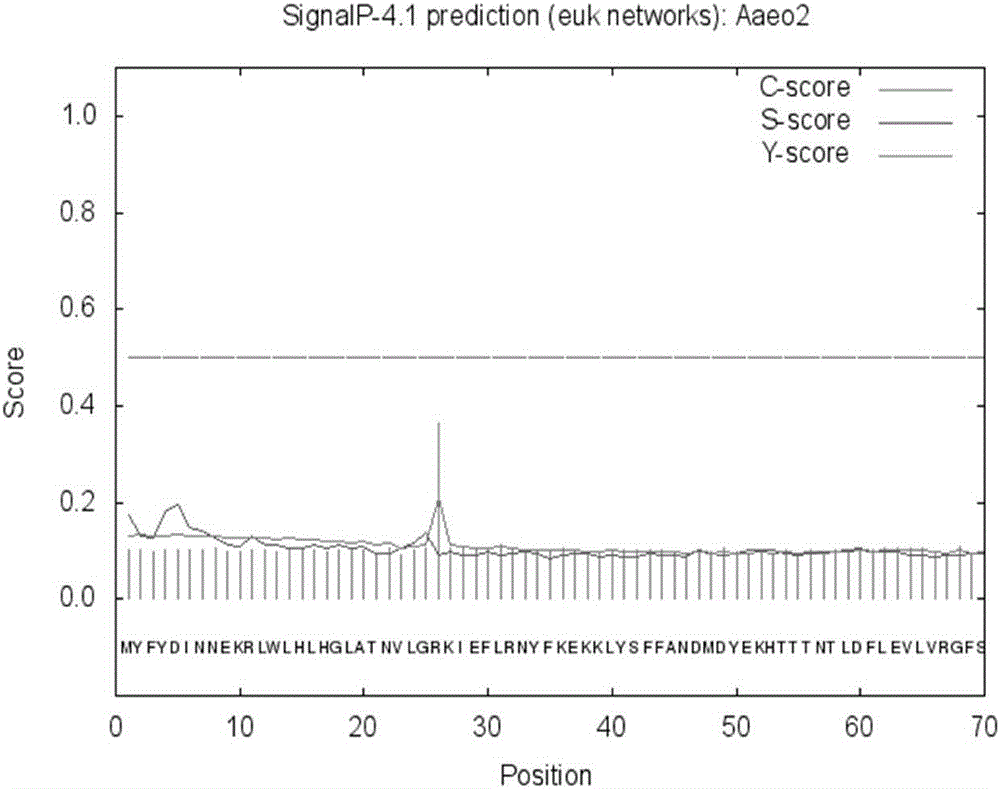
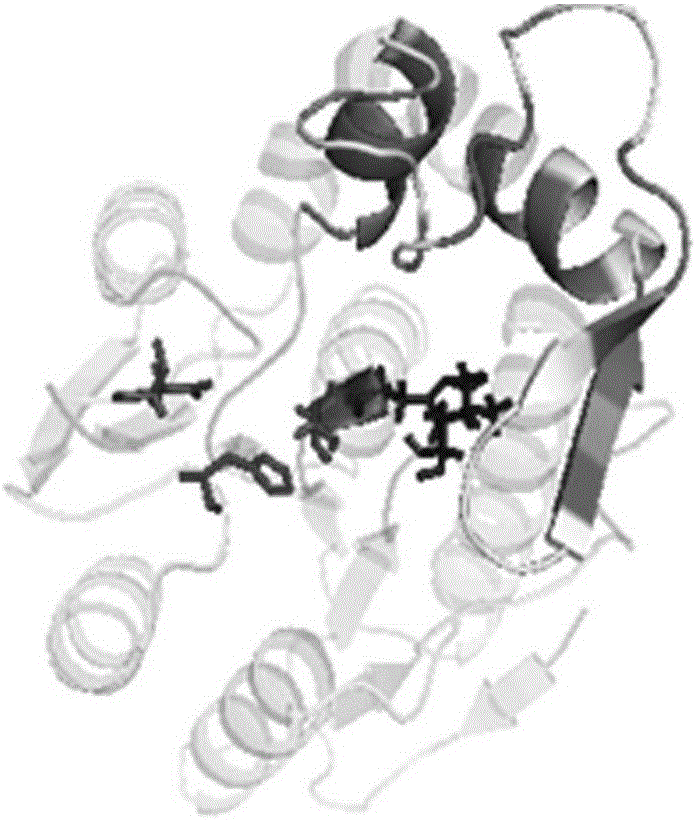
Thermophilic esterase derived from aquifex aeolicus strain and functional verification of thermophilic esterase
The invention discloses thermophilic esterase derived from an aquifex aeolicus strain and functional verification of the thermophilic esterase, and belongs to the field of bioengineering technology. According to the invention, a novel thermophilic esterase gene is discovered, three expression systems are constructed, and efficient expression and research of enzymatic properties are achieved. Construction of an eukaryotic expression system: preferably, a vector pPIC9K is adopted for expression vector construction, and a pichia pastoris host, preferably GS115, is transformed, so that efficient expression is achieved; construction of a prokaryotic escherichia coli expression system: preferably, a vector MBP3 is adopted for expression vector construction, and escherichia coli hosts, preferably BL21 and Origami2, are transformed, so that efficient expression is achieved; and construction of a prokaryotic bacillus megaterium expression system: preferably, a vector pHIS1525 is adopted for expression vector construction, and a bacillus megaterium host, preferably YYBm1, is transformed, so that efficient expression is achieved. The recombinant enzyme (the thermophilic esterase) has the advantages of esterase activity, ,thermophilic characteristic, thermal stability and the like; and the recombinant enzyme has a great potential in industrial application under a high-temperature condition.
Owner:JIANGNAN UNIV
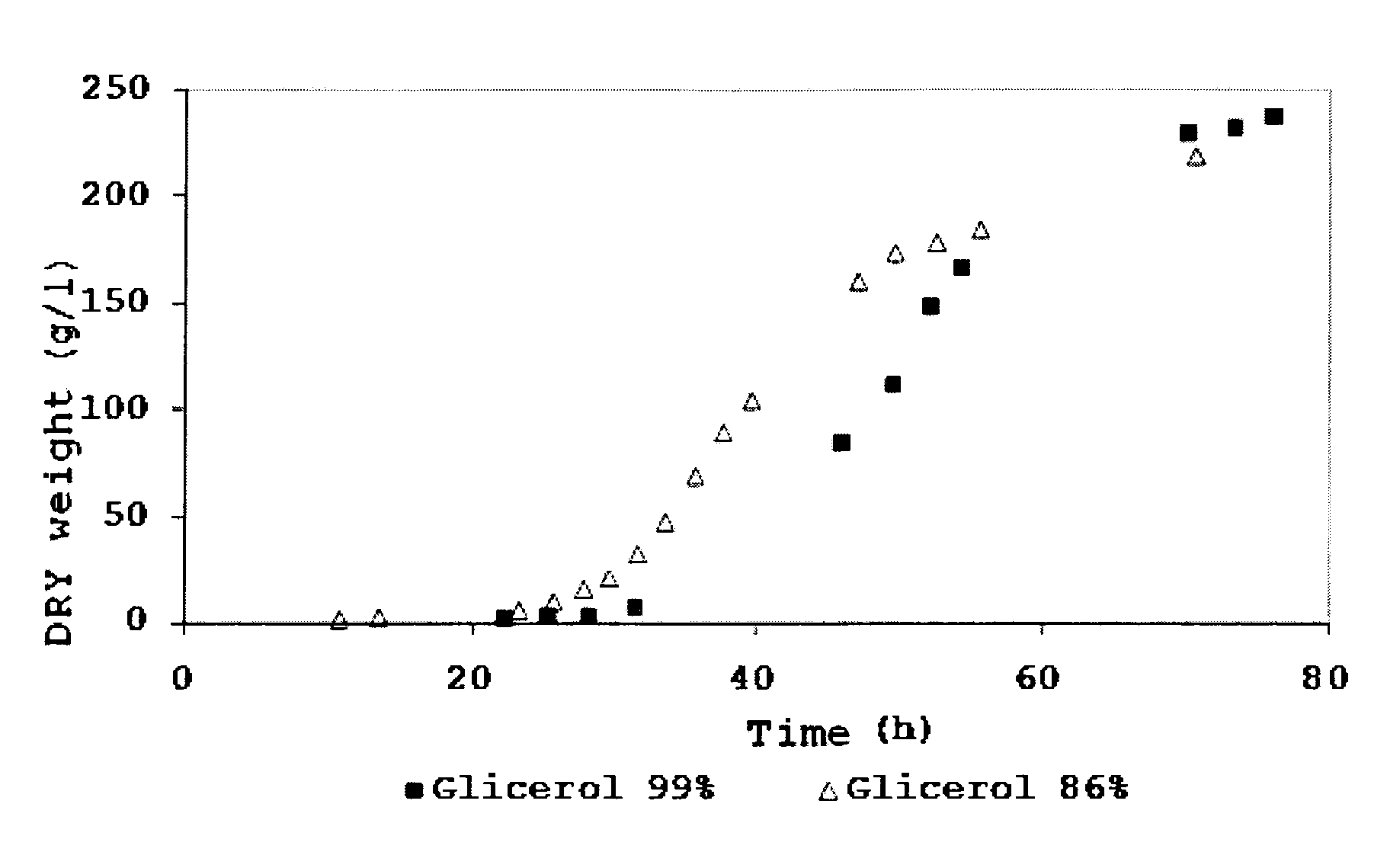
Process for the co-production of chitin, its derivatives and polymers containing glucose, mannose and/or galactose, by the fermentation of the yeast Pichia pastoris
ActiveUS8614070B2Density in maximizedMaximize productivityMicroorganismsFermentationPichia pastorisHigh cell
The presently disclosed subject matter concerns a process for the co-production of glucosamine polymers (chitin, chitosan or any of its derivatives) and polymers containing glucose, mannose and / or galactose, by the high cell density fermentation of the yeast Pichia pastoris in a bioreactor under aerobic conditions. The process can include the use of glycerol byproduct from the biodiesel industry as carbon source. Pure glycerol, pure methanol, glycerol-rich or methanol rich mixtures may also be used as carbon sources. The P. pastoris fermentation process can be duly optimized for attaining high cell densities and high cell wall chitin content. The disclosed subject matter also concerns polymers containing glucose, mannose and / or galactose.
Owner:73100 SETENTA E TRES MIL E CEM LDA
Construction method of Pichia pastoris expressed by OCH1 defect anti-CD20 tetravalent antibody
InactiveCN105779490AInhibition formationNarrowing Glycosylation DifferencesFungiHybrid immunoglobulinsPichia pastorisHigh mannose
The invention discloses a construction method of Pichia pastoris expressed by an OCH1 defect anti-CD20 tetravalent antibody. The method comprises the following steps: knocking out the alpha-1,6-mannosyl transferase OCH1 of a strain JC308, connecting reconstructed och1 having no expression ability and a screening label to pPICZalphaA, introducing the obtained pPICZalphaA to wild Pichia pastoris JC308, carrying out homologous recombination, screening to obtain an OCH1 defect strain denoted as detaoch1, connecting the sequence of a synthesized anti-CD20 tetravalent antibody with a Pichia pastoris expression plasmid pPIC9, and introducing the obtained product to the OCH1 defect strain detaoch1 to obtain the Pichia pastoris. The Pichia pastoris alpha-1,6-mannosyl transferase (OCH1) gene is knocked out to prevent formation of high-mannose carbohydrate chains, so glycosylation difference between yeast expression proteins and human natural proteins is shortened, and the safety of medicinal proteins is guaranteed.
Owner:BEIJING JIZHI XINCHUANG TECH
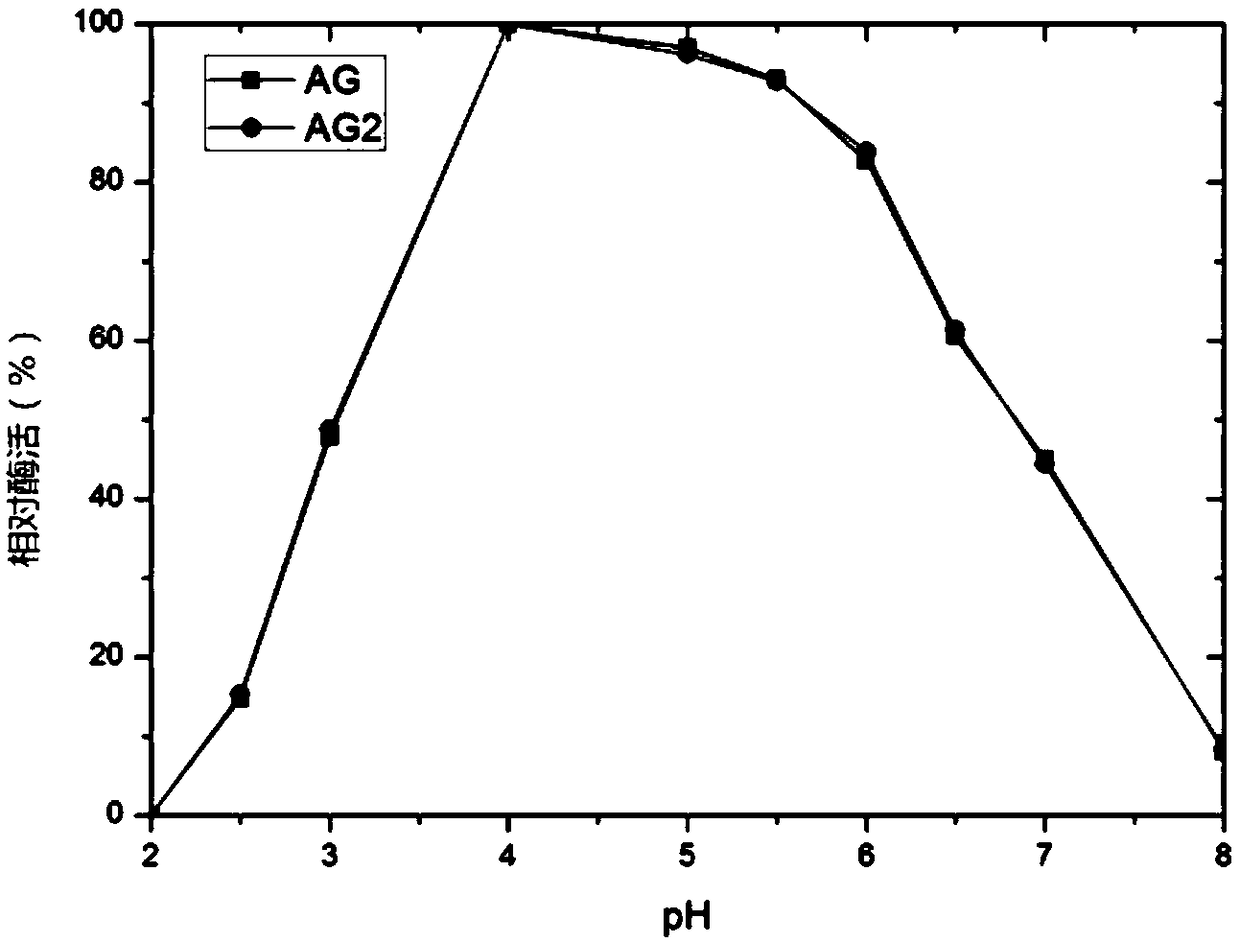
Pichia pastoris strain capable of producing high-yield alpha-galactosidase
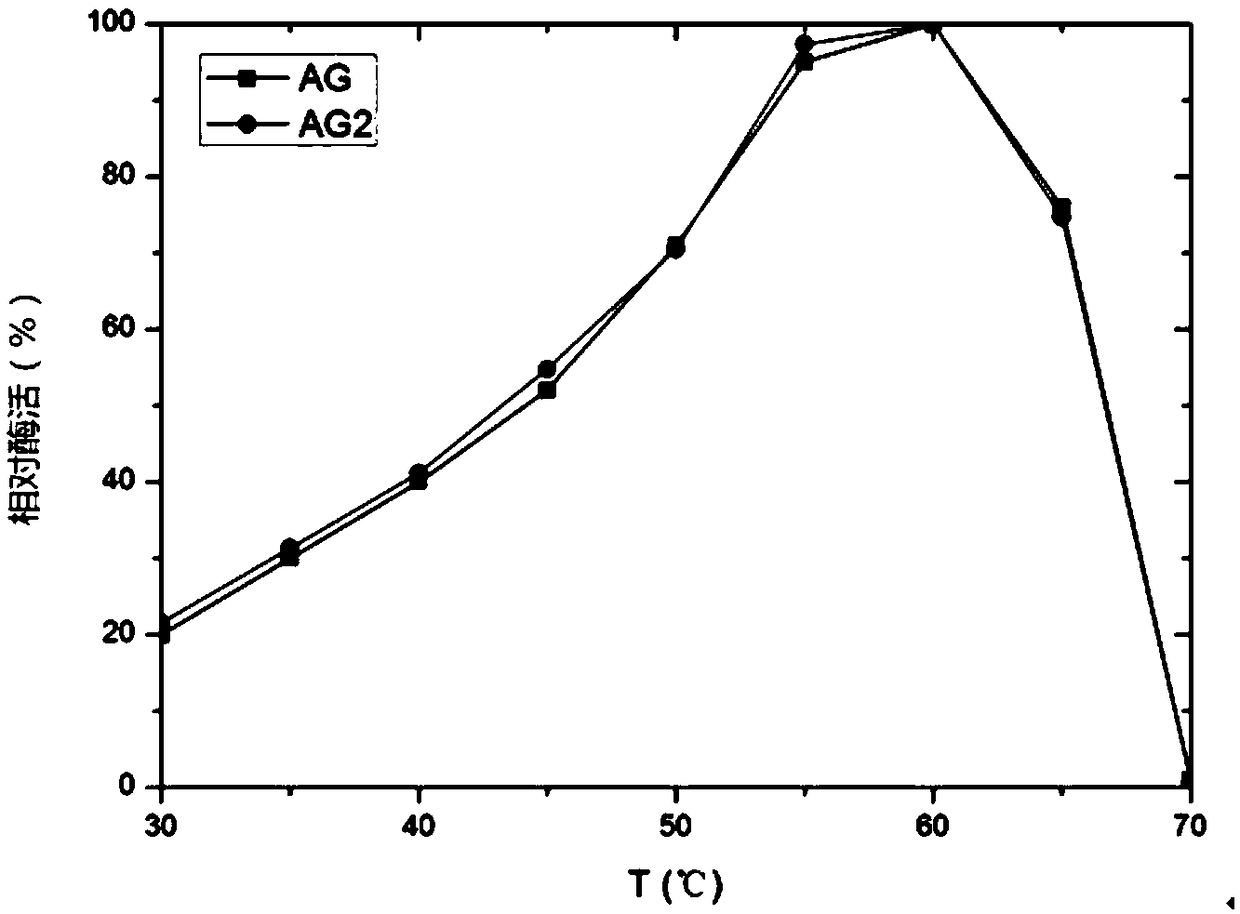
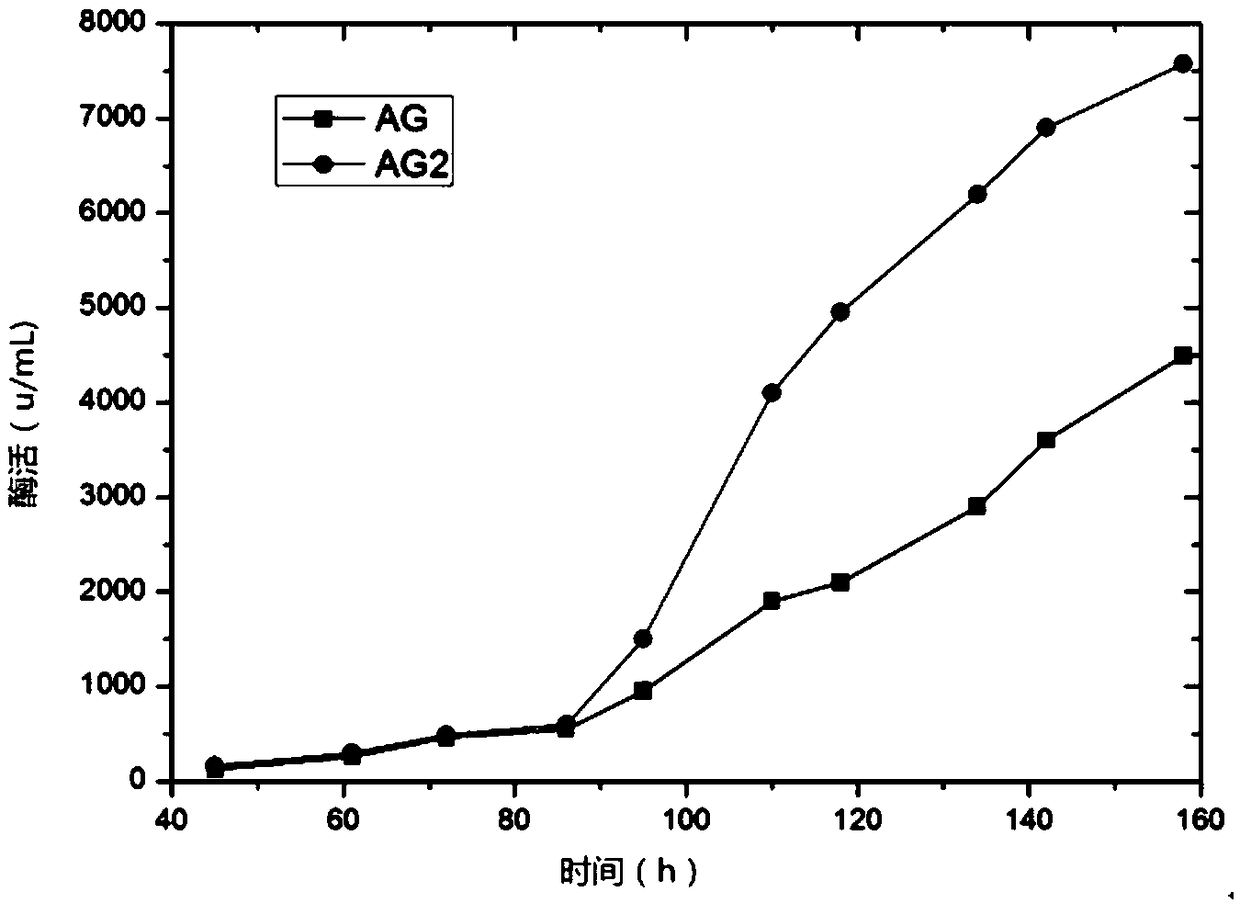
The invention relates to a pichia pastoris mutant strain, wherein the preservation number is CCTCC NO: M2018376. The mutant strain perform efficient recombinant expression on alpha-galactosidase, theenzyme activity of alpha-galactosidase in supernatant fermented by shaking a flask is up to 109 U / mL and is137 percent higher than that of the original strain; the enzyme activity of alpha-galactosidase in supernatant fermented by a 20-L tank is up to 7576 U / mL and is 69 percent higher than that of the original strain and the unexpected technical effect is achieved. Furthermore, compared with theoriginal strain, the enzymatic property of the alpha-galactosidase which is subjected to recombinant expression by the mutant strain pichia pastoris AG2 is not changed, the pH of the moist proper effect is 3 to 7, and the temperature of the most proper effect is 60 DEG C. The mutant strain pichia pastoris AG2 can be widely applied to production of the alpha-galactosidase.
Owner:WEIFANG KANGDIEN BIOTECH +1
Laccase gene derived from laccaria bicolor, and applications thereof
ActiveCN104357414AGreat application potentialFungiMicroorganism based processesPichia pastorisNucleotide
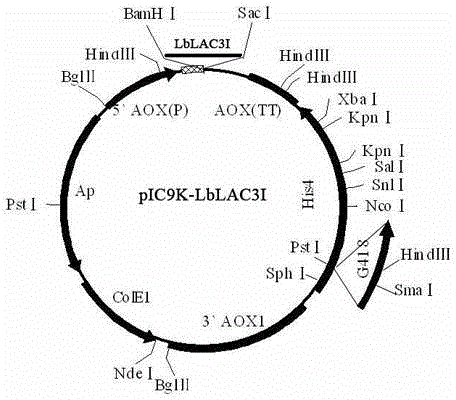
The invention discloses an optimized laccase LbLAC3I sequence applicable to pichia pastoris expression, and expression applications thereof. The nucleotide sequence of the laccase LbLAC3I is shown as SEQIDNo1, the full length is 1518bp, and the coded protein sequence is shown as SEQIDNo2 and totally contains 501 amino acids. The sequence is constructed onto a pichia pastoris expression carrier and transformed into pichia pastoris for expression, thus being used for laccase and decoloration of a dye.
Owner:SHANGHAI ACAD OF AGRI SCI
A production method and applications of a novel rhizomucor miehei aspartic protease
ActiveCN107338234AEffective tenderizationEffective hydrolysisFermentationVector-based foreign material introductionBiotechnologyPichia pastoris
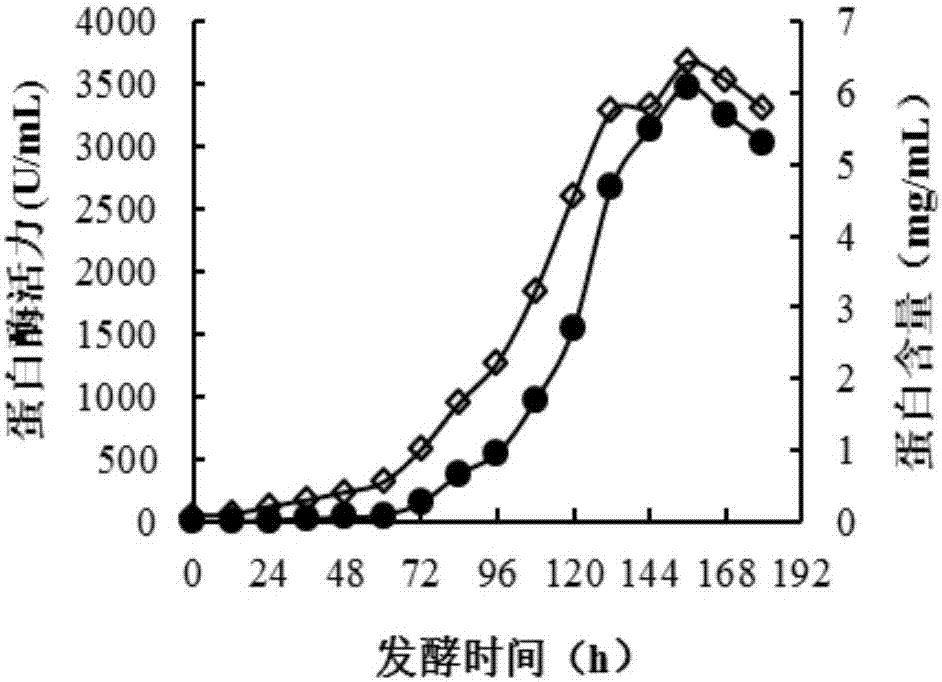
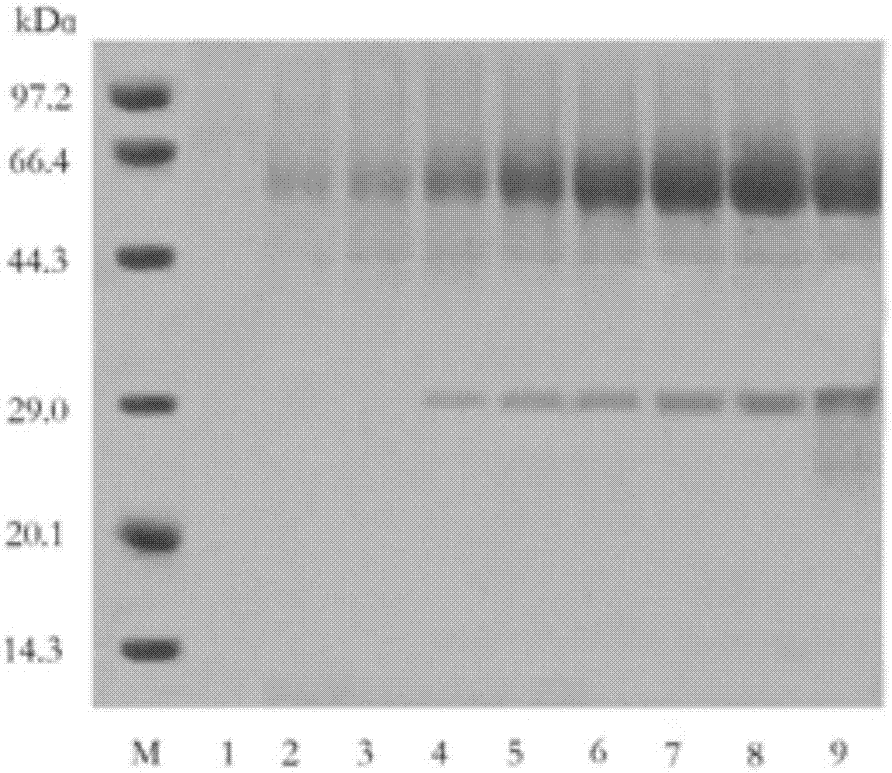
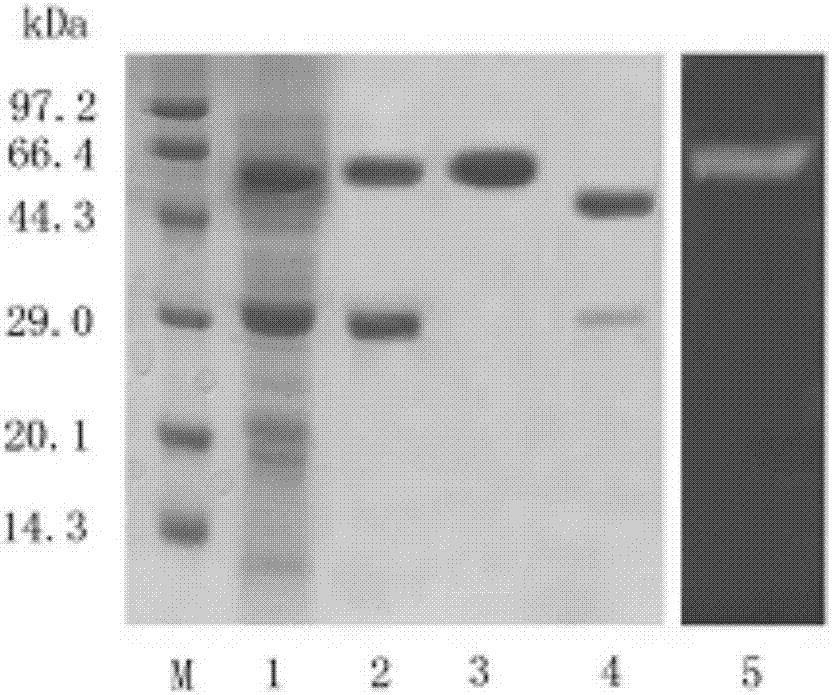
The invention discloses a production method and applications of a novel rhizomucor miehei aspartic protease, and particularly relates to a recombinant aspartic protease, a coding gene, and a production method and applications of the recombinant aspartic protease. The recombinant aspartic protease is protein having an amino acid sequence shown as SEQ ID NO:1. The coding gene of the recombinant aspartic protease is a DNA molecule shown as SEQ ID NO:2. An aspartic protease gene that is RmproA of rhizomucor miehei is connected to a pichia pastoris GS115 expression vector pPIC9K, and is converted into pichia pastoris and induced expression is performed to obtain the recombinant aspartic protease. Through high-density fermentation in a fermentation tank having a volume of 5 L, the maximum protease production activity at 156 h of a recombinant strain is 3400 U / mL, and the protein content is 6.42 mg / mL. The recombinant aspartic protease is capable of effectively tendering pork, reducing shearing force, making meat palatable, and effectively hydrolyzing animal and plant protein to prepare low-molecular-weight polypeptides.
Owner:CHINA AGRI UNIV
Gene segment for coding porcine interferon-gamma and application of gene segment
ActiveCN103014015AImprove biological activityHigh expressionPeptide/protein ingredientsMicroorganism based processesBiotechnologyPichia pastoris
According to the feeding preference of the pichia pastoris expression system codon, a high-expression codon is selected to be redesigned and synthetized into a novel porcine interferon-gamma mature peptide nucleotide sequence. A recombinant yeast expression plasmid pPICZalphaC-IFN-gamma is successfully constructed, so that the correct expression of the porcine interferon-gamma is achieved in the pichia pastoris, the porcine interferon-gamma is expressed more easily in the pichia pastoris, the expression quantity is higher, and the foundation is laid from the large-scale production of gene engineering.
Owner:SOUTH CHINA AGRI UNIV +1
Method for improving expression level of Pichia pastoris recombinant protein
The invention relates to a method for improving the expression level of Pichia pastoris recombinant protein. On the basis of the normal process flow of recombinant Pichia pastoris, trace melatonin is added to greatly improve the expression of the recombinant protein. By utilizing the method, radical accumulation in yeast brought by intimidation, such as unbalanced oxygen supply, during the high-density fermentation of the recombinant saccharomycete is lowered, and the damage on the yeast brought by the radical in the high-density fermentation process is eliminated so as to improve the physiological condition of the yeast. After the melatonin is used, the expression level of the recombinant protein is improved to a large extent.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com