High efficient preparation of novel antimicrobial peptide Mytichitin-A in pichia pastoris
A technology of Pichia pastoris and antimicrobial peptides, which can be applied in the direction of antibacterial drugs, medical preparations containing active ingredients, peptides, etc., can solve the problems of undisclosed use and achieve high-efficiency expression and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
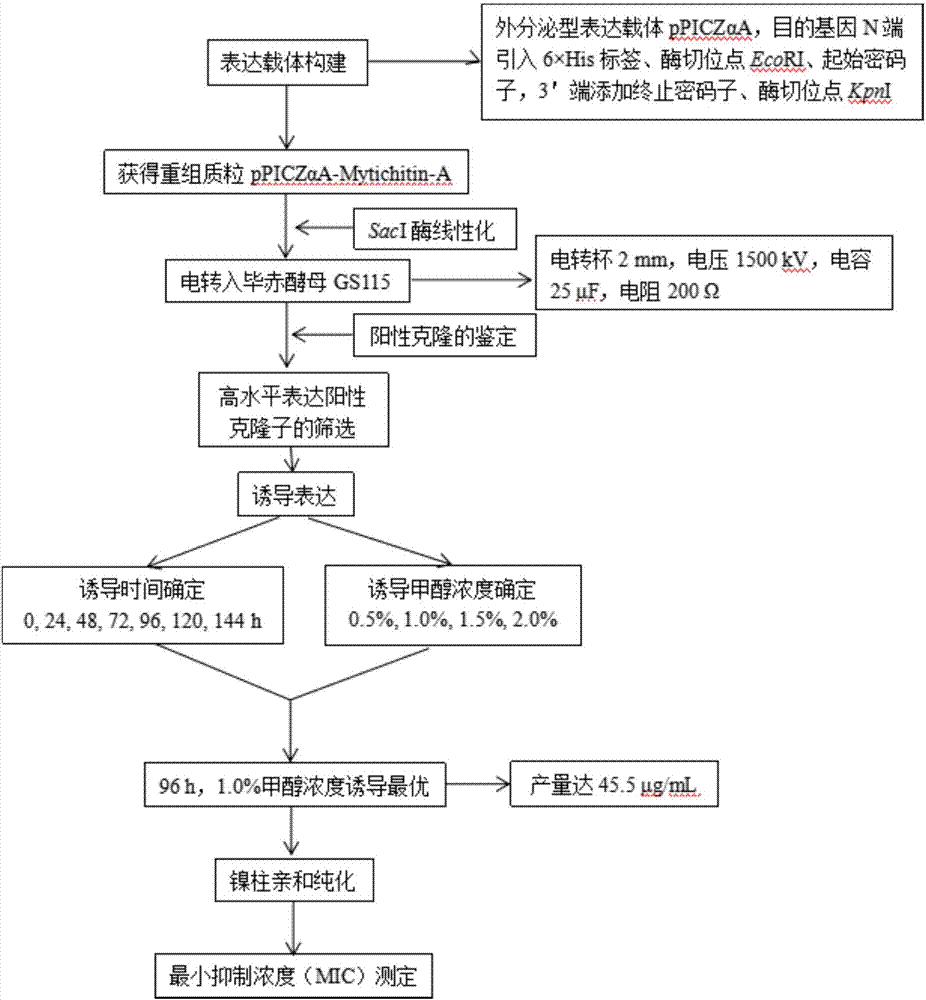
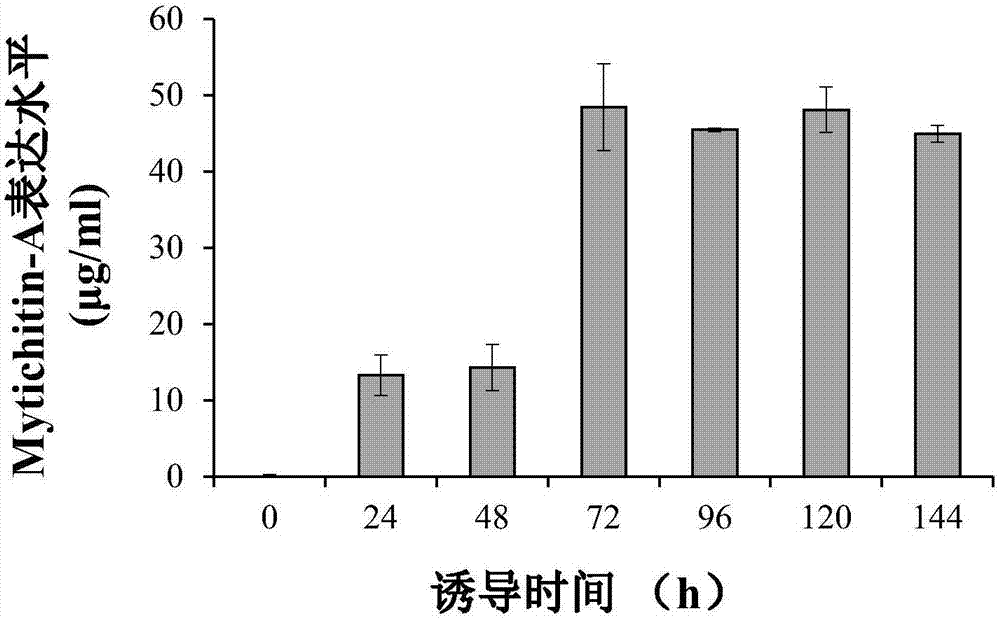
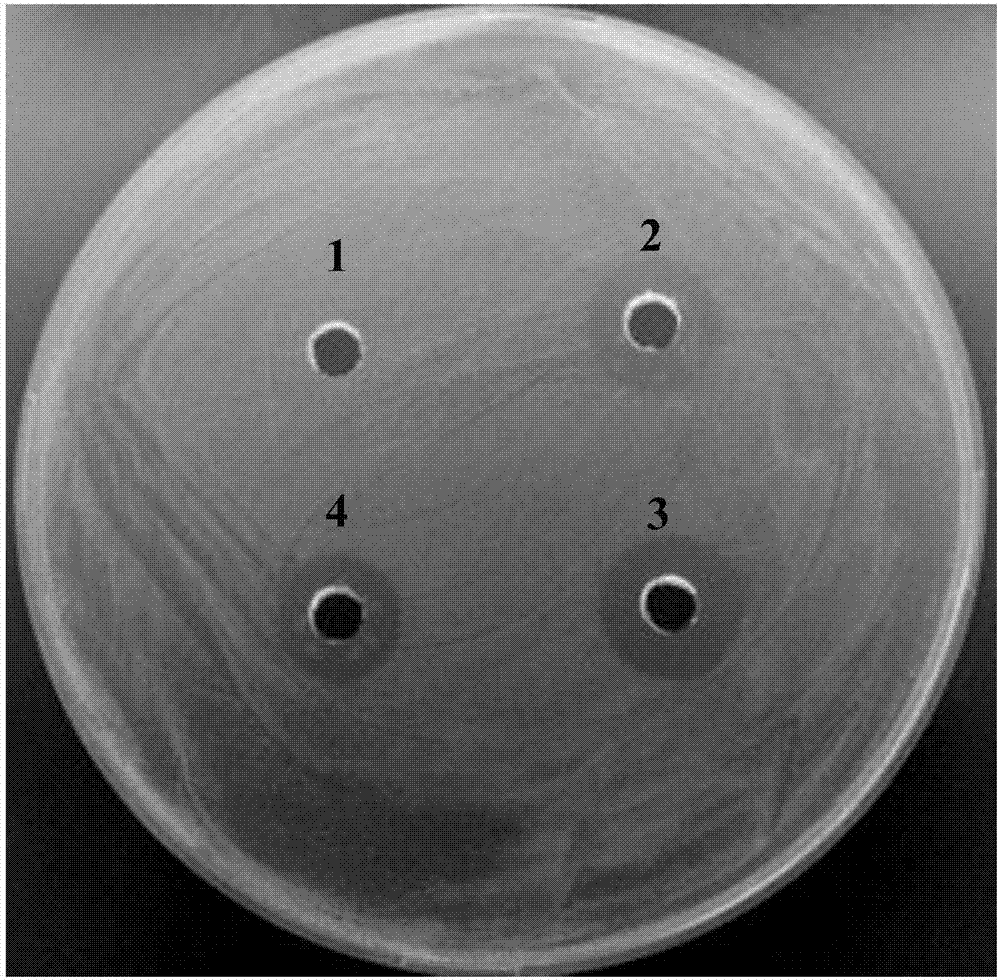
[0024] Construction of expression vector - add 6×His tag, enterokinase gene sequence, EcoRI restriction site and start codon to the 5' end of the target gene Mytichitin-A; introduce stop codon and KpnI restriction site at 3' point, according to SEQ ID NO: 2, the whole gene synthesis was carried out, and inserted into the vector pUC19 to obtain the recombinant plasmid pUC-Mytichitin-A. The recombinant pUC-Mytichitin-A and pPICZαA empty vectors were subjected to double enzyme digestion (EcoRI, KpnI) respectively, at 37°C for 3h. Enzyme digestion system is 10×Buffer 1μL, pUC-Mytichitin-A / pPICZαA 1.5μg, EcoRI 0.8μL, KpnI 0.8μL, total system 10μL; recover the ligation, target gene Mytichitin-A fragment and vector pPICZαA fragment are ligated for 2h at room temperature or 4℃ Overnight ligation, ligation system: pPICZαA 20ng, Mytichitin-A 8ng, T4 ligase 0.8μL, total system 10μL. The pPICZα-Mytichitin-A recombinant plasmid was successfully constructed. The recombinant plasmid pPICZα...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com