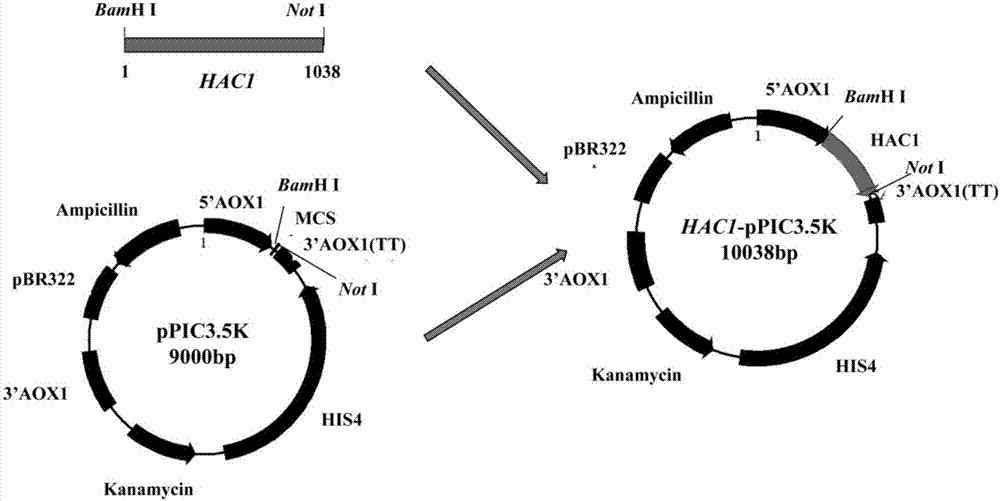
Recombinant pichia pastoris for heterogenous efficient expression of lipase and application of recombinant pichia pastoris
A lipase, heterologous technology, applied in the biological field, can solve the problems of secretion efficiency, high enzyme activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 Rhizomucor miehei cDNA
[0026] 1.1 Extraction of Rhizomucor militaris total RNA
[0027] (1) Take an appropriate amount of Rhizomucor miehei hyphae, absorb the water with filter paper, grind with liquid nitrogen, add 1ml Trizol reagent (Invitrogen), vibrate with an oscillator for 5 minutes, and let stand at room temperature for 1 minute;
[0028] (2) Add 0.2ml chloroform, shake for 15s, and let it stand for 2min;
[0029] (3) 4°C, 12000rpm, 15min;
[0030] (4) Aspirate the supernatant, add an equal volume of isopropanol, and precipitate at -20°C for 30 minutes;
[0031] (5) 4°C, 12000rpm, 15min;
[0032] (6) Pour off the supernatant, wash the precipitate with 1ml 75% ethanol, 7500rpm, 4°C, 5min;
[0033] (7) Repeat (6) step once;
[0034] (8) Pour off the supernatant and dry for 10 minutes;
[0035] (9) Add appropriate amount of DEPC water to dissolve to obtain total RNA;
[0036] 1.2 Preparation of the first strand of Rhizomucor ...
Embodiment 22
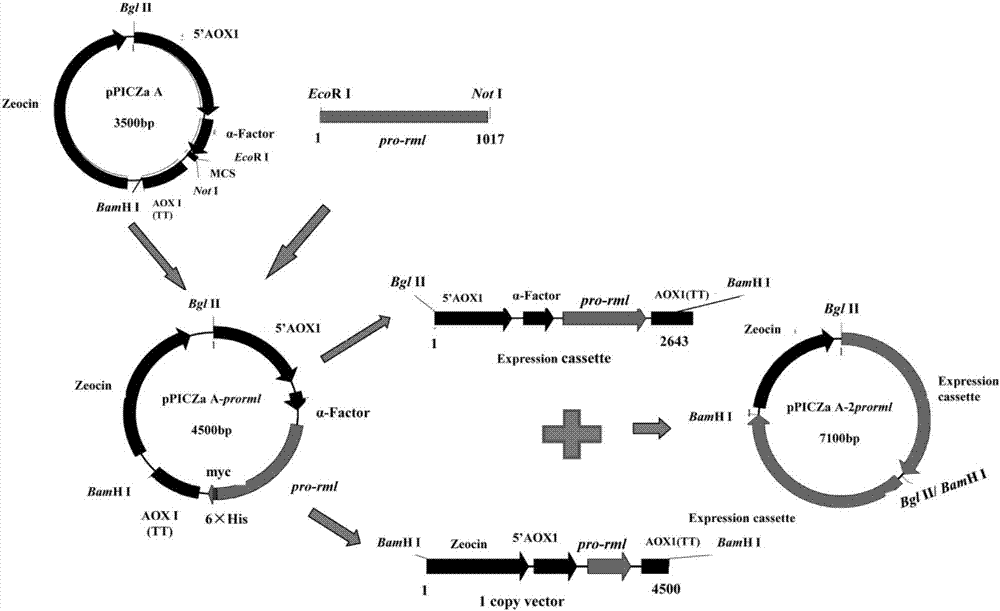
[0041] Example 2 Construction of 2 copies of pPICZα A-2prorml plasmid
[0042] 2.1 Primer design
[0043] According to the sequence of the rml gene in GenBank (GenBank accession number is A02536.1), the following pair of primers were designed and synthesized:
[0044] FW(P1): 5'—CG GAATTC GTGCCAATCAAGAG—3' (SEQ ID NO.2)
[0045] REV (P2): 5'-TAG TCTAGAGTACAGAGGCCTGTG-3' (SEQ ID NO.3)
[0046] EcoR I and Not I restriction sites are designed at both ends of P1 and P2 respectively (see the italicized and underlined part in the above sequence)
[0047] 2.2 PCR amplification of Rhizomucor miehei lipase pro-rml containing leader peptide
[0048] Using P1 and P2 primers, Rhizomucor miehei (Boel E, Huge-Jensen B, Christensen M, Thim L, Fiil N: Rhizomucor miehei triglyceride lipase is synthesized as aprecursor.Lipids 1988,23(7):701-706. ) cDNA as a template, the PCR reaction system is:
[0049]
[0050] The reaction conditions are: 95°C for 5min, 5°C for 40s, 60°C for 40s, ...
Embodiment 3
[0057] Example 3 Screening of Pichia pastoris recombinant strains with 4 copies of pro-rml gene
[0058] 3.1 Preparation of Pichia pastoris X-33 (purchased by Invitrogen) electroporation competent cells
[0059] (1) Pick a fresh single colony and put it in 5ml YPD liquid medium, cultivate it at 30°C and 250rpm for 12-14h;
[0060] (2) Inoculate 0.1% of the inoculum into a 2L Erlenmeyer flask containing 500ml of YPD medium, cultivate at 30°C and 250rpm for 12-14h to make OD 600 =1.3-1.5;
[0061] (3) Centrifuge at 1500 rpm for 5 minutes at 4°C to collect the cells;
[0062] (4) Wash the cells twice with 500-250ml ice-cold sterile water;
[0063] (5) Wash the cells once with 20ml of ice-cold 1M sorbitol solution;
[0064] (6) Resuspend the cells with 1ml of ice-cold 1M sorbitol solution to a final volume of about 1.5ml, and dispense 80μl into small centrifuge tubes;
[0065] 3.2 Electric shock transformation of Pichia pastoris yeast cells
[0066] (1) Mix about 10 μl of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com