Multi-copy high expressed recombined plectasin by pichia pastoris
A technique of lectin and expression cassette, which is applied in the field of the preparation of a recombinant lectomycin gene multi-copy expression vector and its recombinant yeast, can solve the problem of limited expression amount, large workload of screening multi-copy, low multi-copy gene, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The construction of embodiment 1 plectasin genetically engineered bacteria
[0036] 1.1 Design of Plectasin gene based on yeast preferred codons
[0037] The Plectasin gene was artificially designed according to the codon usage preference of Pichia pastoris (http: / / www.kazusa.or.jp / codon / ), and the obtained sequence is shown in SEQ ID NO.1.
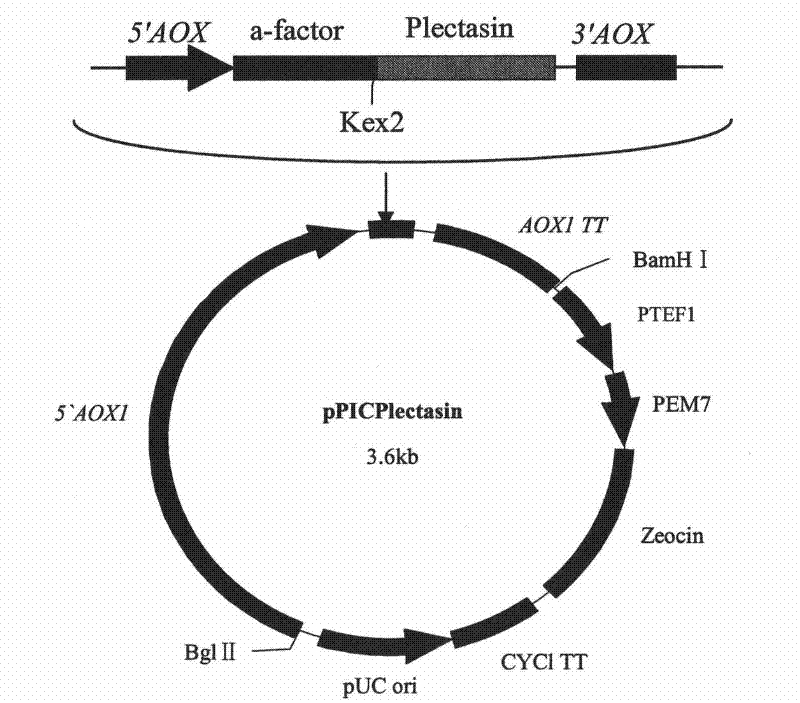
[0038] 1.2 Construction of single-copy expression vector of recombinant plectasin
[0039] At the 5'-end of the Plectasin gene, a restriction endonuclease XhoI cleavage site, which is not available in the Plectasin gene but on the multiple cloning site of the vector, is designed, and the yeast Kex2 cleavage site after the XhoI cleavage site is retained (KR) coding sequence, in order to cleave the signal peptide after secretion and expression, to obtain recombinant Plectasin, design the TAATAA terminator sequence and XbaI restriction site at the 3'-end of the gene, in order to terminate the expression of the polypeptide and constru...
Embodiment 2
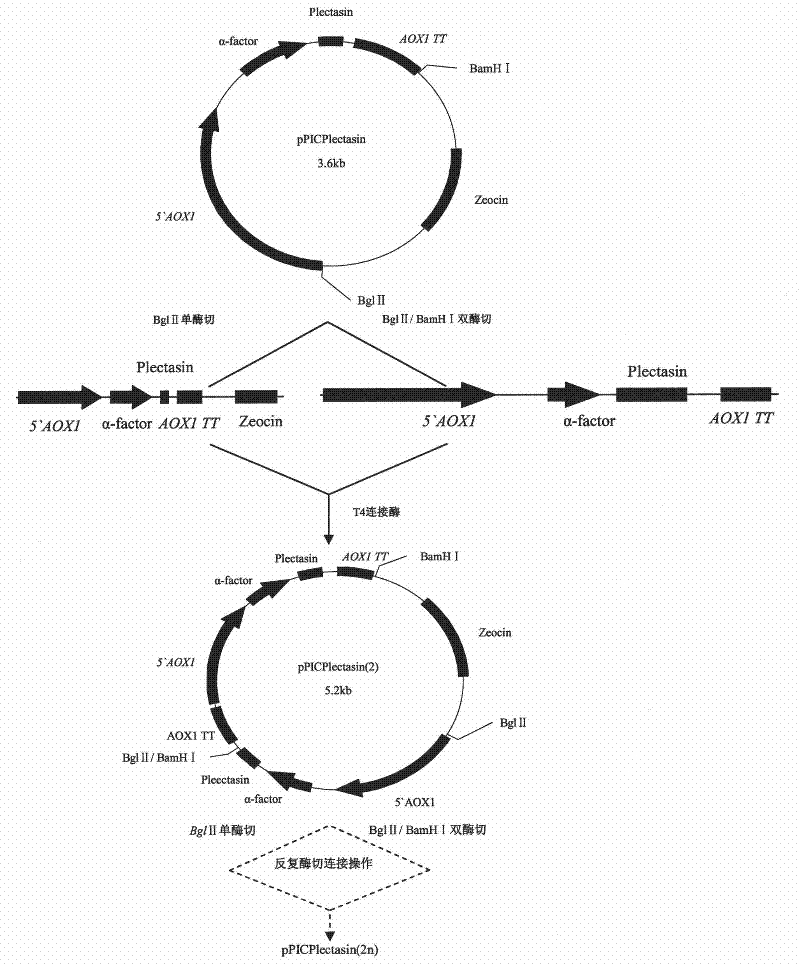
[0071] Induced expression of embodiment 2 recombinant plectasin
[0072] 1% of the inoculum was inoculated into 10 mL of BMGY medium with two copies, four copies, and eight copies of the correctly identified plectasin, shaken to OD at 30°C and 250 rpm 600nm 4.0 (logarithmic growth phase, about 16-18h); centrifuge at 2500g for 5min at room temperature. Discard the supernatant and resuspend the cells to OD with 50mL BMMY medium 600nm 1.0; add the above culture into a 250mL Erlenmeyer flask, cover with 4 layers of sterilized gauze, and put it in a shaker to continue to grow; every 24 hours, add methanol to a final concentration of 0.5% to continue induction; at the following time points, 0h , 24h, 48h, 72h, 96h, 120h Take 1mL of medium to a 1.5mL centrifuge tube and measure the OD value at this time point, centrifuge at 12000rpm at room temperature for 2-3min, and the induction time is 120h. Dilute the bacterial suspension to the same OD value, centrifuge to collect the superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com