Reductively degradable mercaptopurine nanometer micellar prodrug with controllable drug release and application thereof
A nanomicelle, mercaptopurine technology, applied in the fields of nanomedicine, new materials, and biomedical technology, can solve problems such as unfavorable long-acting circulation, lack of biocompatibility, unable to achieve stable release, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
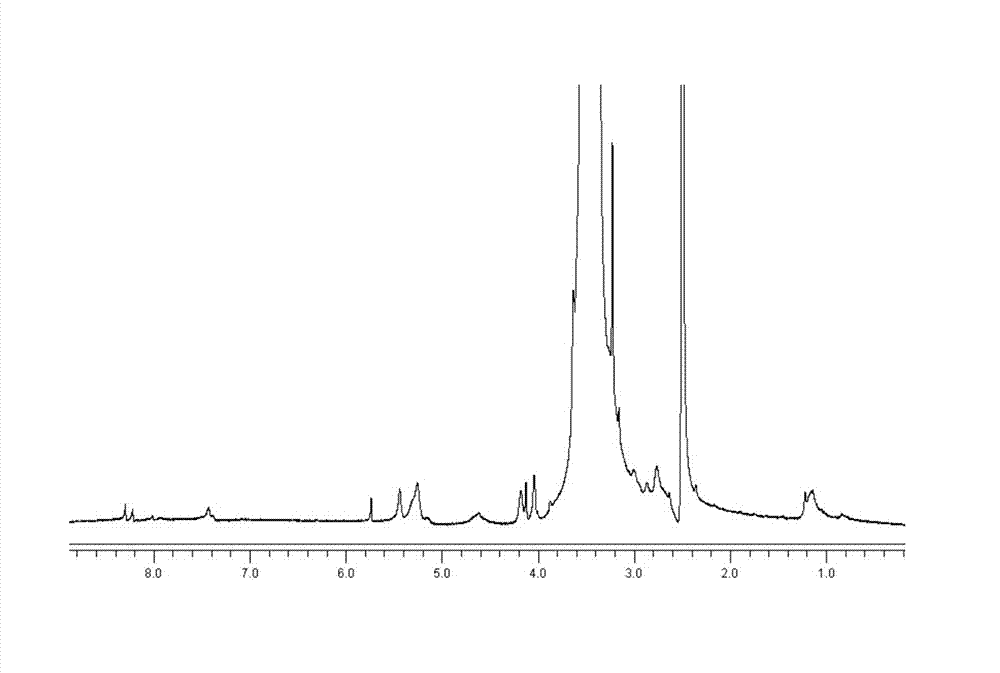
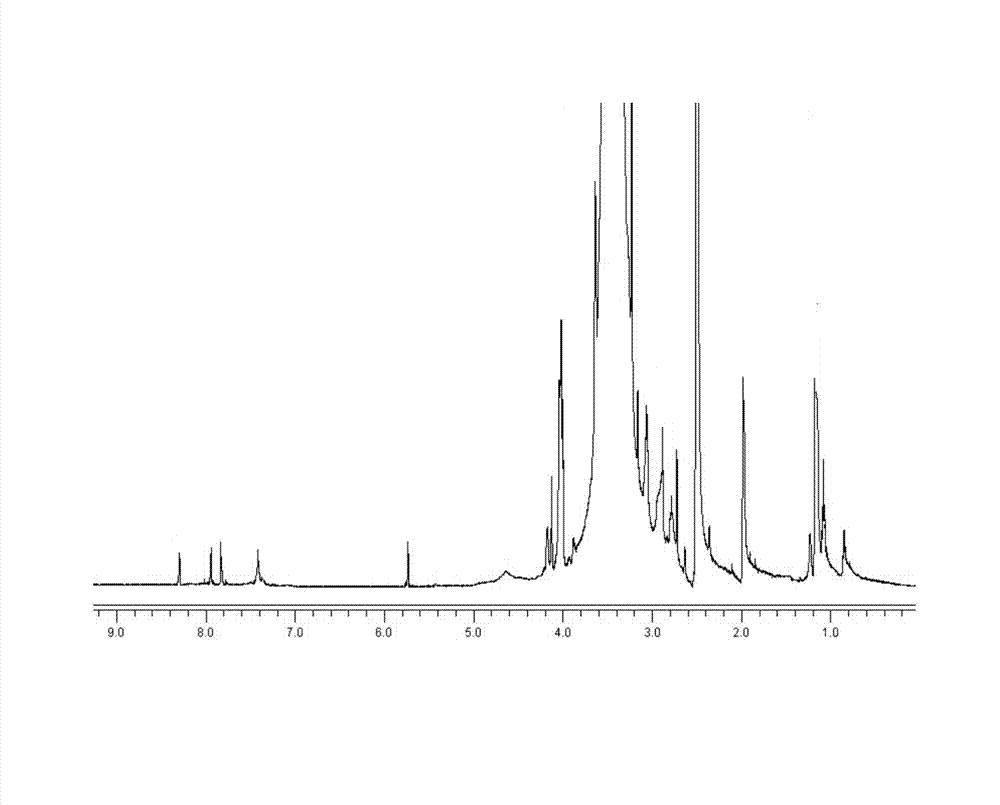
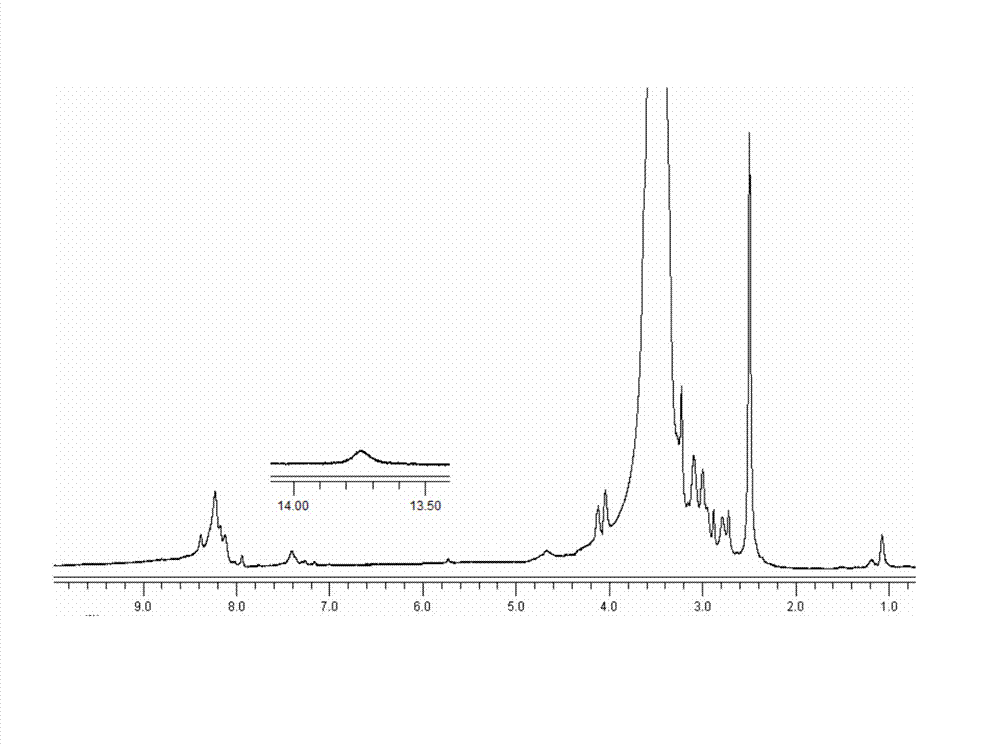
Image
Examples
Embodiment 1
[0043]Preparation of mercaptopurine nanomicelle prodrug of the present invention
[0044] The first step, the synthesis of mPEG-NPC
[0045] 1.1) Dissolve mPEG (Mw= 1900, 7.6 g, 4 mmol) and p-nitrophenyl chloroformate (NPC, 3.21 g, 16 mmol) in 30 mL of dichloromethane (DCM);
[0046] 1.2) Add 0.81 mL (10 mmol) pyridine to the DCM solution of mPEG, under nitrogen protection at room temperature, drop NPC into the mPEG solution drop by drop, and stir for 24 h;
[0047] 1.3) Concentrate the reaction solution and reprecipitate three times in diethyl ether, then vacuum dry to obtain mPEG-NPC.
[0048] The second step, mPEG-SS-NH 2 Synthesis
[0049] 2.1) Dissolve cystamine dihydrochloride (2.89 g, 12.88 mmol) and triethylamine (4.21 mL, 30 mmol) in dimethyl sulfoxide (DMSO), and gradually drop into the first step product mPEG-NPC (3.8 g, 1.84 mmol) in DMSO solution, continued stirring for 24 hours;
[0050] 2.2) Use a dialysis bag with a molecular weight cut-off of 1000 to dial...
Embodiment 2
[0068] Assembly of targeted mercaptopurine nanomicelle prodrugs
[0069] Weigh 20 mg of lyophilized mPEG-SS-NH-g-PAsp-MP and dissolve in 10 mL of deionized water. After ultrasonic oscillation, amphiphilic nanomicelles are formed, and the particle size measured by dynamic light scattering is 160 nm. ( Figure 6 ), belonging to the nanometer level.
Embodiment 3
[0071] Targeting the degradation of mercaptopurine nanomicelle prodrugs
[0072] A sufficient amount of dithiothreitol (DTT) was added to the above-mentioned micelles to carry out reductive degradation of the micelles, and 1 mL of samples were taken at intervals, and the change in particle size was measured by DLS. like Figure 7 As shown, with the increase of time, the particle size of the micelles gradually increased, indicating that under the reduction of DTT, the disulfide bonds were broken, and the micelles were degraded and agglomerated to increase the particle size.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com