Nickel-cobalt based catalyst as well as preparation method and application thereof
A catalyst, a nickel-cobalt-based technology, applied in the field of nickel-cobalt-based catalysts and their preparation, can solve problems such as gas blockage and disasters, and achieve the effects of cost reduction, strong carbon deposition resistance, and good reaction stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 3.62g of cerium nitrate and dissolve in water to form cerium nitrate aqueous solution; dry 10.00g of γ-Al at room temperature 2 o 3 Immerse in the prepared cerium nitrate aqueous solution for 12 hours by equal volume impregnation method; dry at 120°C for 24 hours; bake at 450°C for 2 hours in air atmosphere to obtain the modified carrier.
[0028] Weigh 1.19g nickel nitrate and 1.77g cobalt nitrate and dissolve in water to make a mixed aqueous solution of nickel nitrate and cobalt nitrate; 12 hours in the mixed aqueous solution; drying at 120° C. for 24 hours; calcining at 700° C. for 5 hours in an air atmosphere to obtain a catalyst precursor.
[0029] The prepared catalyst precursor was reduced in hydrogen at 900 °C for 2 hours.
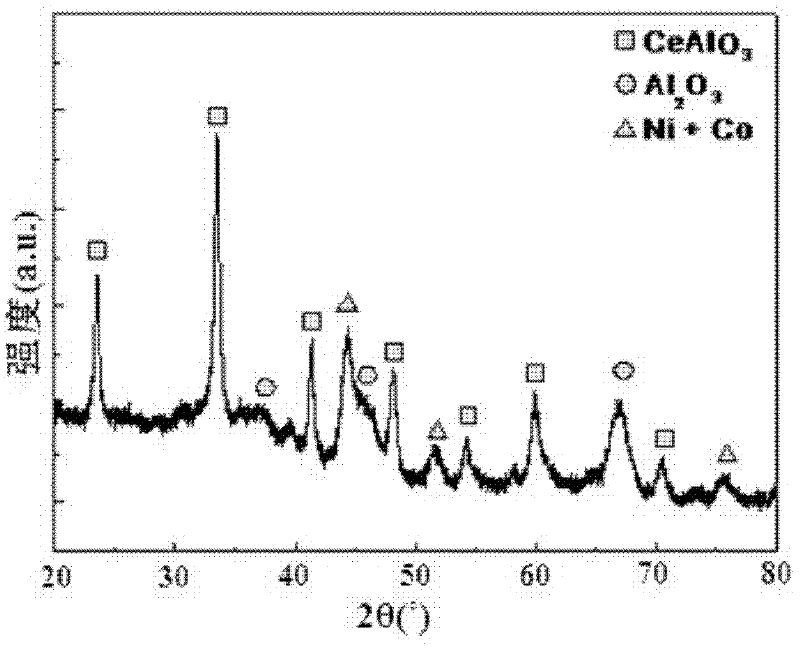
[0030] figure 1 For the XRD collection of collections of catalysts that the present embodiment makes, by figure 1 It can be seen that the prepared catalyst is composed of four phases of metal nickel, metal cobalt, cerium aluminate ...
Embodiment 2
[0037] Weigh 3.46g of cerium nitrate and dissolve in water to form cerium nitrate aqueous solution; dry 10.00g of γ-Al at room temperature 2 o 3 Immerse in the prepared cerium nitrate aqueous solution for 12 hours by equal volume impregnation method; dry at 120°C for 24 hours; bake at 450°C for 2 hours in air atmosphere to obtain the modified carrier.
[0038] Weigh 0.28g nickel nitrate and 0.28g cobalt nitrate and dissolve in water to make a mixed aqueous solution of nickel nitrate and cobalt nitrate; 12 hours in the mixed aqueous solution; drying at 120° C. for 24 hours; calcining at 700° C. for 5 hours in an air atmosphere to obtain a catalyst precursor.
[0039] The prepared catalyst precursor was reduced in hydrogen at 900 °C for 2 hours.
[0040] The XRD spectrum of the catalyst prepared in this example shows that the prepared catalyst is composed of four phases of metallic nickel, metallic cobalt, cerium aluminate and aluminum oxide.
[0041] After X-ray diffraction ...
Embodiment 3
[0047] Weigh 4.42g of cerium nitrate and dissolve in water to form cerium nitrate aqueous solution; dry 10.00g of γ-Al at room temperature 2 o 3 Immerse in the prepared cerium nitrate aqueous solution for 12 hours by equal volume impregnation method; dry at 120°C for 24 hours; bake at 450°C for 2 hours in air atmosphere to obtain the modified carrier.
[0048] Take by weighing 10.84g nickel nitrate and 3.60g cobalt nitrate and dissolve in water and be made into the mixed aqueous solution of nickel nitrate and cobalt nitrate; 12 hours in the mixed aqueous solution; drying at 120° C. for 24 hours; calcining at 700° C. for 5 hours in an air atmosphere to obtain a catalyst precursor.
[0049] The prepared catalyst precursor was reduced in hydrogen at 900 °C for 2 hours.
[0050] The XRD spectrum of the catalyst prepared in this example shows that the prepared catalyst is composed of four phases of metallic nickel, metallic cobalt, cerium aluminate and aluminum oxide.
[0051] A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com