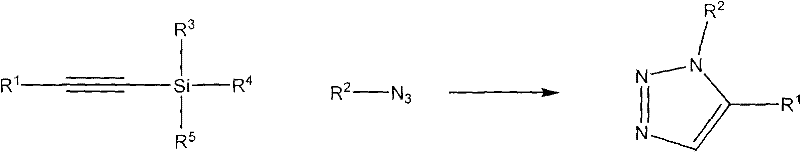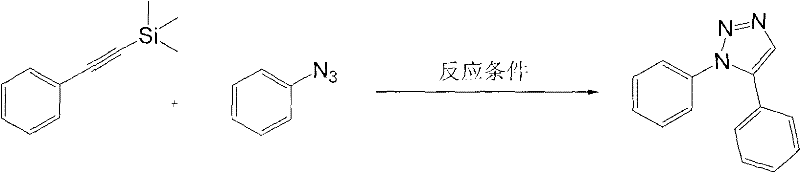Method for synthesizing 1,2,3-triazole compounds by utilizing sila-alkyne compounds
A technology of silicon-based alkynes and compounds, which is applied in the field of preparation of triazole compounds, can solve problems such as limitations, and achieve the effects of avoiding the use of heavy metals, simple process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of 1,5-diphenyl-1,2,3-triazole
[0019]
[0020] Add 174 mg (1 mmol) of trimethylsilylphenylacetylene, 119 mg (1 mmol) of phenyl azide, and 2 mL of dimethyl sulfoxide into the reactor, keep the reaction temperature at 30 ° C, and add the desilication reagent KOH into the reaction system 73mg (1.3mmol), start stirring, and continue to react until thin plate chromatography shows that the raw material disappears. Then 6 mL of saturated ammonium chloride solution and 50 mL of ethyl acetate were added, and the mixture was transferred to a separatory funnel, washed four times with 50 mL of water, and once with 20 mL of saturated brine. Anhydrous Na for organic phase 2 SO 4 dry. Filtration, removal of solvent under reduced pressure and column chromatography yielded the title compound. Yield, 85%. The NMR data are as follows:
[0021] 1 H NMR (CDCl 3 , 400MHz): δ7.19-7.24(m, 2H), 7.30-7.38(m, 5H), 7.40-7.46(m, 3H), 7.86(s, 1H); 13 C NMR (CDCl 3 , 100MHz...
Embodiment 2
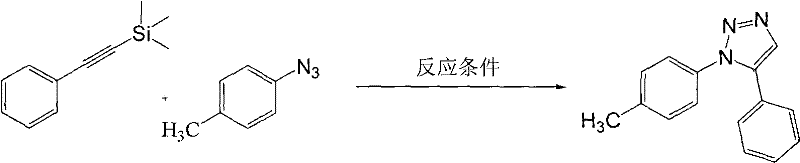
[0023] Preparation of 1-(4-methylphenyl)-5-phenyl-1,2,3-triazole
[0024]
[0025] Add 174 mg (1 mmol) of trimethylsilylphenylacetylene, 134 mg (1 mmol) of 4-methylphenyl azide, and 2 mL of dimethyl sulfoxide into the reactor, keep the reaction temperature at 30 ° C, and add Silicon-based reagent KOH 73mg (1.3mmol), start stirring, and continue the reaction until thin plate chromatography shows that the raw material disappears. Then 6 mL of saturated ammonium chloride solution and 50 mL of ethyl acetate were added, and the mixture was transferred to a separatory funnel, washed four times with 50 mL of water, and once with 20 mL of saturated brine. Anhydrous Na for organic phase 2 SO 4 dry. Filtration, removal of solvent under reduced pressure and column chromatography yielded the title compound. Yield, 83%. The NMR data are as follows:
[0026] 1 H NMR (CDCl 3 , 400MHz): δ2.35(s, 3H), 7.09-7.18(m, 4H), 7.35-7.42(m, 2H), 7.43-7.46(m, 3H), 7.83(s, 1H); 13 C NMR (CDCl...
Embodiment 3
[0028] Preparation of 1-(4-methoxyphenyl)-5-phenyl-1,2,3-triazole
[0029]
[0030] Add 174 mg (1 mmol) of trimethylsilylphenylacetylene, 150 mg (1 mmol) of 4-methoxyphenyl azide, and 2 mL of dimethyl sulfoxide into the reactor, keep the reaction temperature at 30 ° C, and add Desilication reagent KOH 73mg (1.3mmol), start stirring, continue to react until the thin plate chromatography shows that the raw material disappears. Then 6 mL of saturated ammonium chloride solution and 50 mL of ethyl acetate were added, and the mixture was transferred to a separatory funnel, washed four times with 50 mL of water, and once with 20 mL of saturated brine. Anhydrous Na for organic phase 2 SO 4 dry. Filtration, removal of solvent under reduced pressure and column chromatography yielded the title compound. Yield, 80%. The NMR data are as follows:
[0031] 1 H NMR (CDCl 3 , 400MHz): δ3.83(s, 3H), 6.92(d, J=2H), 7.21-7.30(m, 4H), 7.31-7.38(m, 3H), 7.85(s, 1H); 13 C NMR (CDCl 3, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com