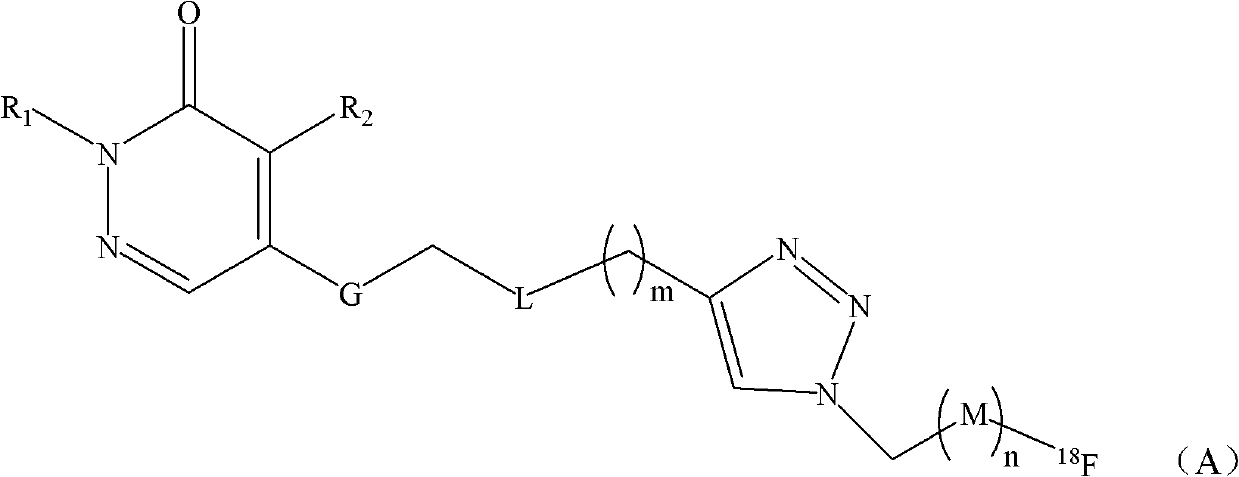
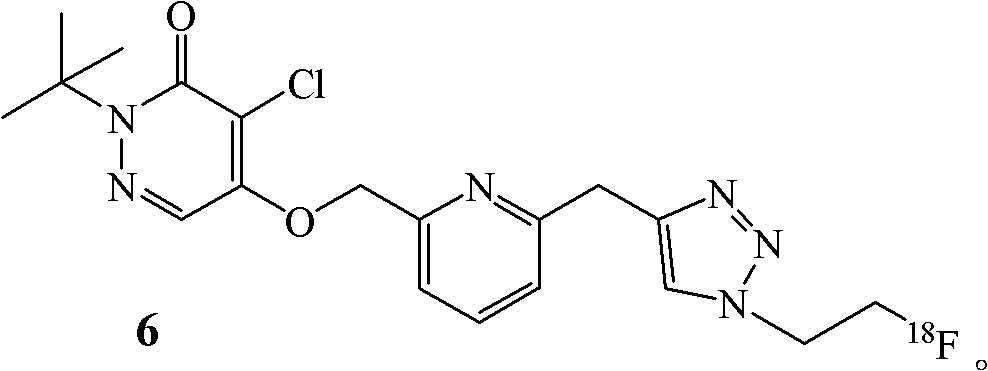
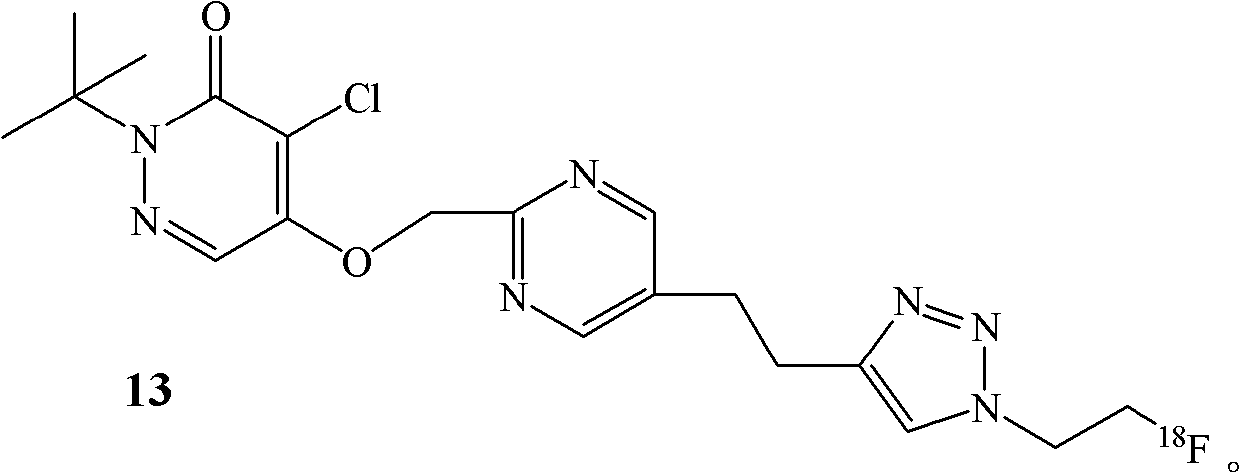
Fluorine-18-marked myocardial perfusion developing agent and preparation method and application thereof
A technology of labeling and pyridazinone, which is applied in the field of radiopharmaceutical chemistry and clinical nuclear medicine, and can solve the problems of long waiting time for imaging, poor stability of aqueous solution, complex synthetic route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Preparation of labeled compound 6
[0029] The synthetic route is as follows:
[0030]
[0031] (1) Synthesis of labeled precursor compound 4.
[0032] a. Synthesis of Compound 1
[0033]Add 1.392g of 2,6-dihydroxypyridine, 1.258g of 4,5-dichloropyridazinone, 3.256g of cesium carbonate and 10mL of anhydrous N,N-dimethylformamide into a 50mL eggplant-shaped bottle at 68°C Oil bath reaction 12h. The reaction solution was cooled to room temperature, diluted with 50 mL of ethyl acetate, and filtered with suction. The filtrate was transferred to a 100mL separatory funnel and washed 4 times with 50mL water respectively. The organic phase was dried over anhydrous magnesium sulfate, and after suction filtration, the filtrate was rotary distilled and purified through a 200-300 mesh silica gel column. The developing solvent was petroleum ether: ethyl acetate = 1:1. Compound 1 was finally obtained as a white solid. NMR spectrum: NMR spectrum: ( 1 HNMR, CDCl 3...
Embodiment 2
[0046] Example 2. Preparation of labeled compound 13
[0047]
[0048] (1) Synthesis of precursor compound 11:
[0049] a. Synthesis of Compound 8
[0050] Add 1.543g of compound 7, 1.258g of 4,5-dichloropyridazinone, 3.256g of cesium carbonate and 10mL of anhydrous N,N-dimethylformamide into a 50mL eggplant-shaped bottle, and react in an oil bath at 68°C for 12h. The reaction solution was cooled to room temperature, diluted with 50 mL of ethyl acetate, and filtered with suction. The filtrate was transferred to a 100mL separatory funnel and washed 4 times with 50mL water respectively. The organic phase was dried over anhydrous magnesium sulfate, and after suction filtration, the filtrate was rotary distilled, and purified through a 200-300 mesh silica gel column. The developing solvent was petroleum ether: ethyl acetate = 1:2. Compound 8 was finally obtained as a white solid. NMR spectrum: ( 1 HNMR, CDCl 3 )δ: 1.477(s, 9H, N(CH 3 ) 3 ), 2.801(t, 2H, CH 2 CH 2 OH),...
Embodiment 3
[0063] Example 3. Preparation of labeled compound 16
[0064]
[0065] (1) Synthesis of labeled precursor compound 14:
[0066] Add 0.125g of copper sulfate pentahydrate and 0.396g of sodium L-ascorbate into a 50mL flask, add 2mL of water under nitrogen protection, and stir for 15min. Dissolve 0.254 g of 1-ethoxy-2-propynyl p-toluenesulfonate in 1.5 mL of N,N-dimethylformamide and add to the reaction flask. 0.363 g of compound 10 was dissolved in 1.5 mL of N,N-dimethylformamide and added to the reaction flask. Stir at room temperature for 1 d under airtight conditions. Add 50 mL of dichloromethane. The solution was transferred to a 250 mL separatory funnel and washed with 50 mL saturated brine. The organic phase was dried with anhydrous magnesium sulfate, and after suction filtration, the filtrate was rotary distilled and purified through a 200-300 mesh silica gel column, and the developing solvent was petroleum ether: ethyl acetate = 1:7. Compound 14 was finally obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com