Alkyne-containing quinoxalin derivative and preparation method thereof
A technology for alkyne quinoxaline and derivatives, which is applied in the field of alkyne-containing quinoxaline derivatives and their preparation, achieving the effects of less environmental pollution, less side reactions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
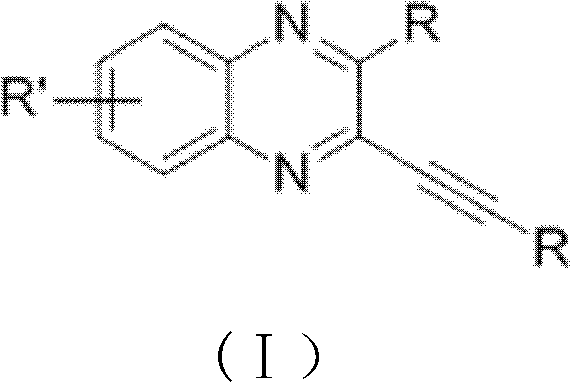
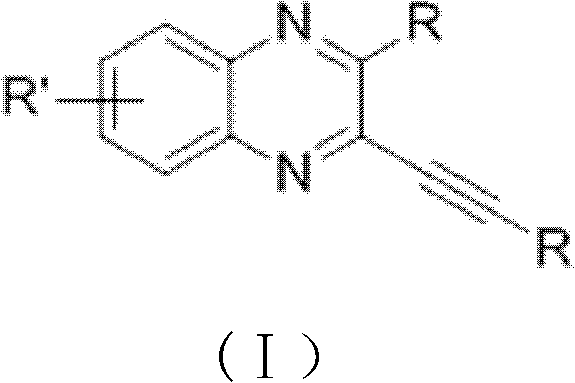
[0036] The general formula of the preparation method of alkyne-containing quinoxaline derivatives of the present invention is as follows:
[0037]
[0038] in:
[0039] R is aryl (for example: phenyl, alkylphenyl, bromophenyl, fluorophenyl, chlorophenyl, alkoxyphenyl, thienyl, pyridyl, naphthyl, etc.), alkyl (for example: hexyl , butyl, propyl, tert-butyl, etc.) or other groups (for example: trimethylsilyl, haloalkyl, etc.).
[0040] R' is hydrogen, halogen, aryl (for example: phenyl, alkylphenyl, bromophenyl, fluorophenyl, chlorophenyl, alkoxyphenyl, thienyl, pyridyl, naphthyl, etc.) or Alkyl (for example: hexyl, butyl, propyl, tert-butyl, etc.).
[0041] CuX is CuCl, CuBr or CuI.
Embodiment 1
[0043] Example 1: Preparation and structural characterization of 2-(4-ethylphenyl)-3-(2-(4-ethylphenyl)ethynyl)quinoxaline (a)
[0044] Add 1 mmol of p-ethylphenylacetylene and 5 mmol of o-phenylenediamine into the sealed tube, add 0.2 mmol of catalyst CuCl and 2 ml of solvent chlorobenzene, react for 10 hours in an oil bath at 70 ° C under magnetic stirring, filter, and the filtrate is vortexed by vacuum distillation. The solvent was removed, the residue was dissolved in 10 mL of ethyl acetate, and then washed with water (3×2 mL) and saturated brine (2 mL) successively, the organic layer was dried over anhydrous sodium sulfate, and the ethyl acetate was removed under reduced pressure, and the obtained crude product was coated with silica gel. Column chromatography, with eluent (V 石油醚 :V 乙酸乙酯=60:1), the eluent was evaporated to dryness, and the obtained yellow oil was compound a. Its hydrogen spectrum, carbon spectrum and structural formula are as follows:
[0045] 1 HNMR ...
Embodiment 2
[0048] Example 2: Preparation and structural characterization of 2-hexyl-3-(1-octynyl)quinoxaline (b)
[0049] Add 1mmol of 1-octyne and 3mmol of o-phenylenediamine into the sealed tube, add catalyst 0.3mmol CuI, react in a water bath at 90°C under magnetic stirring for 16 hours, filter, and dissolve the filtrate in 10mL of ethyl acetate and then water (3× 2 mL), washed with saturated brine (2 mL), dried over anhydrous sodium sulfate, and then removed ethyl acetate under reduced pressure. The resulting crude product was subjected to silica gel column chromatography, and the eluent (V 石油醚 :V 乙酸乙酯 =50:1), the eluent was evaporated to dryness, and the obtained brownish-red oil was compound b. Its hydrogen spectrum, carbon spectrum and structural formula are as follows:
[0050] 1 H NMR (500MHz, CDCl 3 )δ8.05-7.95(m, 2H), 7.68(m, 2H), 3.20-3.10(t, J=5.0Hz, 2H), 2.56(t, J=7.1Hz, 2H), 1.85(m, 2H ), 1.76-1.59(m, 2H), 1.56-1.41(m, 4H), 1.41-1.29(m, 8H), 0.91(t, J=5.0Hz, 3H), 0.90...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com