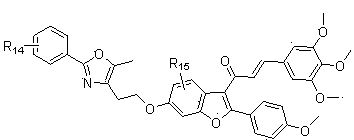
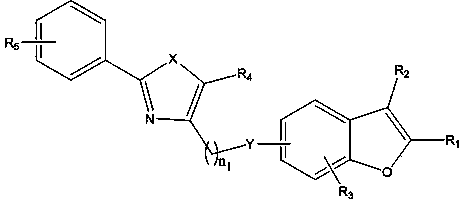
Coumarone compound, its preparation method and its application
A kind of technology of benzofuran and compound is applied in the field of benzofuran compound and its preparation and use, and can solve the problems such as less research on PPAR agonist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
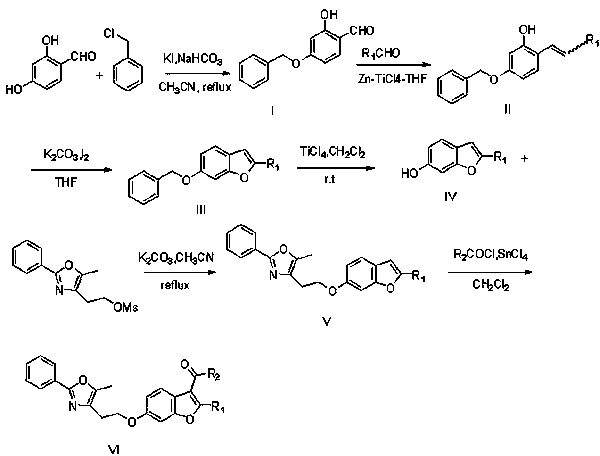
[0040] Synthesis of 4-(benzyloxy)-2-hydroxybenzaldehyde I ( figure 1 ): Dissolve 2,4-dihydroxybenzaldehyde (100mg, 0.72 mmol) in acetonitrile (15ml), then add potassium iodide (179.3mg, 1.08mmol) and sodium bicarbonate (90.7 mg, 1.08mmol), after adding , slowly dropwise added benzyl chloride (100 ul, 0.87mmol), and refluxed for 12 hours. After the reaction was completed, it was quenched with water, extracted with ethyl acetate, combined organic phases, washed three times with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, purified by silica gel chromatography (petroleum ether: ethyl acetate=10 : 1) Obtain 110 mg of 4-(benzyloxy)-2-hydroxybenzaldehyde (colorless solid, yield 67%). 1 HNMR (CDCl 3 ;300MHz), δ5.12(s, 2H, O CH 2 Ph) 6.40-6.64(m, 2H, ArH), 7.41-7.43 (m, 6H, ArH), 9.73(s, 1H, CHO), 11.44(brs, 1H, OH).
Embodiment 2
[0042] (E)-5-(Benzyloxy)-2-(4-methoxystyryl)phenol II 1 Synthesis( figure 1 ): Under nitrogen atmosphere, add zinc powder (1.4g, 22mmol) into anhydrous tetrahydrofuran (20ml), then lower the temperature of the reaction system to -5~0°C, and add titanium tetrachloride dropwise at this temperature (1.2ml, 11mmol), after the addition, the temperature of the reaction system was raised to room temperature, stirred for half an hour, and then refluxed for 2.5 hours. After the reflux, the temperature of the reaction system was lowered to -5~0°C again, and 4-(benzyloxy)-2-hydroxybenzaldehyde (1g, 4.4mmol) and p-methoxybenzaldehyde (721mg, 5.3mmol) of tetrahydrofuran solution, refluxed for 2 hours after dropping. After the reaction was completed, the reaction was quenched with 10% aqueous sodium bicarbonate solution, then extracted with dichloromethane, the organic phases were combined, washed three times with saturated brine, dried over anhydrous sodium sulfate, concentrated under re...
Embodiment 3
[0044] 6-(Benzyloxy)-2-methoxybenzofuran III 1 Synthesis( figure 1 ): Dissolve (E)-5-(benzyloxy-2-(4-methoxystyryl)phenol (280mg, 0.84mmol) in tetrahydrofuran (15ml), add anhydrous potassium carbonate (695 mg, 5.04mmol), stirred for 10 minutes, added iodine (1.28g, 5.04mmol), and stirred at room temperature for 12 hours. After the reaction ended, quench the reaction with saturated aqueous sodium bicarbonate solution, then add dropwise saturated aqueous sodium bisulfite solution to remove Remove residual iodine, then extract with ethyl acetate, combine the organic phases, wash three times with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and purify through silica gel chromatography (petroleum ether:ethyl acetate=5:1 ), to obtain 150 mg of 6-(benzyloxy)-2-methoxybenzofuran (yellow solid, yield 54%). 1 HNMR (CDCl 3 ;300MHz), δ3.87(s, 3H, OCH 3 ), 5.14(s, 2H, O CH 2 Ph), 6.826(s, 1H, ArH), 6.889-6.918(d, 1H, ArH, J=8.7Hz), 6.968-6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com