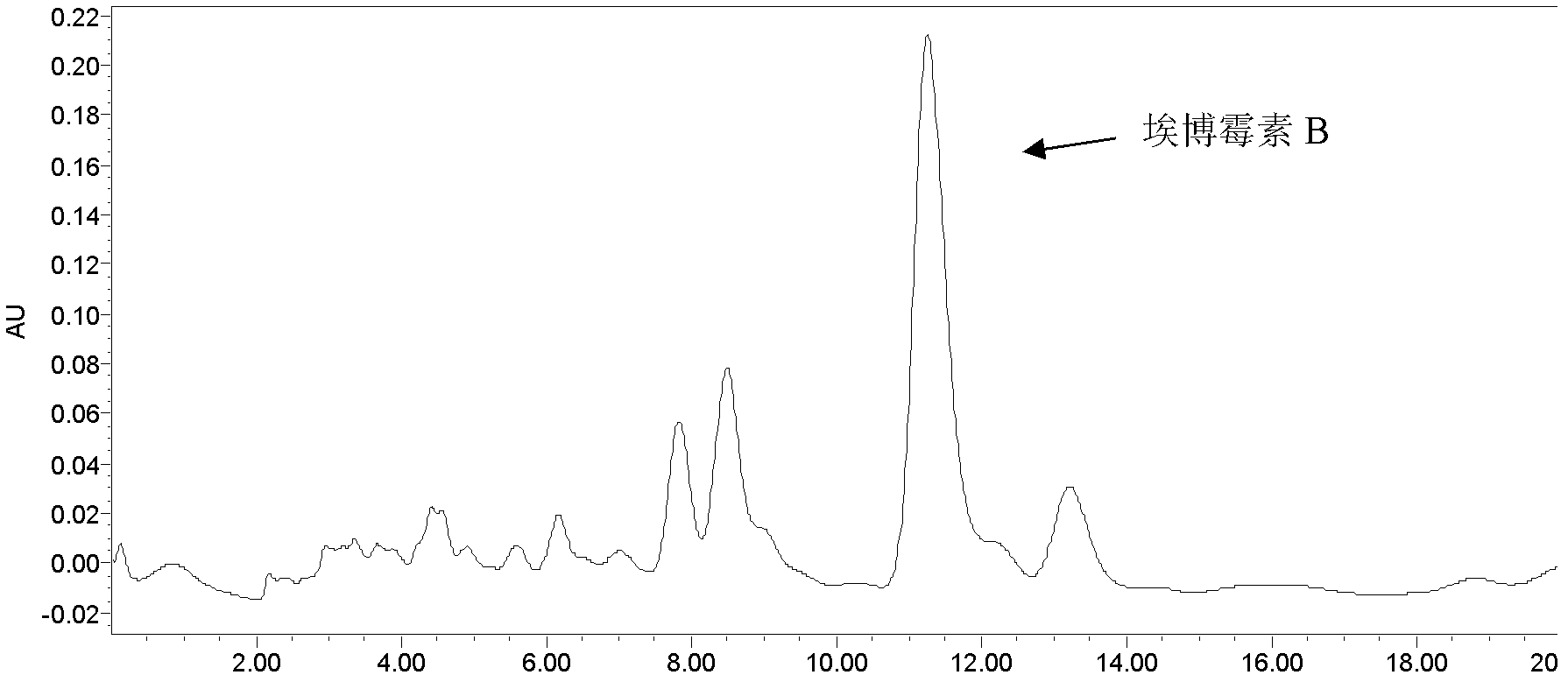
Fermentation production process of Epothilone B
A technology for epothilone and fermentation broth, applied in the directions of fermentation, microorganisms, microorganism-based methods, etc., can solve the problem of high production cost of epothilone, difficulty in increasing epothilone fermentation yield, and high cost of separation and purification of target products. The problem is to eliminate the glucose inhibitory effect and improve the yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of molecularly imprinted polymer and the fermentation of epothilone B include the following steps:
[0031] 1) Preparation of molecularly imprinted polymers
[0032] According to the following proportions: mix 1 mmol of epothilone B, 4 mmol of methacrylic acid, 20 mmol of ethylene glycol dimethacrylate, 10 mg of azobisisobutyrocyanide, and 3 ml of acetonitrile into a reaction tube, pass nitrogen and oxygen for 30 minutes, and Under the protection of nitrogen, the reaction tube was sealed, placed in a water bath at 50°C for 24 hours, and then polymerized in a water bath at 60°C for 12 hours to obtain a polymerization reaction product;
[0033] Crushing, grinding, and sieving the obtained reaction product to obtain polymer particles with a particle size of 40-60 μm;
[0034] After the obtained polymer particles were washed with water, they were extracted with a Soxhlet extractor in a mixed solution of acetic acid:methanol (volume ratio 1:9) for 48 hours, ...
Embodiment 2
[0049] The preparation of molecularly imprinted polymer and the fermentation of epothilone B include the following steps:
[0050] 1) Preparation of molecularly imprinted polymers
[0051] According to the following proportions: mix 1 mmol of epothilone B, 3 mmol of methacrylic acid, 25 mmol of ethylene glycol dimethacrylate, 10 mg of azobisisobutyronitrile, and 8 ml of methanol into a reaction tube, pass nitrogen and exhaust oxygen for 30 minutes, and Under the protection of nitrogen, the reaction tube was sealed, placed in a 45°C water bath for 24 hours of polymerization, and then polymerized in a 65°C water bath for 12 hours to obtain a polymerization reaction product;
[0052] Crushing, grinding, and sieving the obtained reaction product to obtain polymer particles with a particle size of 40-60 μm;
[0053] After the obtained polymer particles were washed with water, they were extracted with a Soxhlet extractor in a mixed solution of acetic acid:methanol (volume ratio 3:7...
Embodiment 3
[0065] The preparation of molecularly imprinted polymer and the fermentation of epothilone B include the following steps:
[0066] 1) Preparation of molecularly imprinted polymers
[0067] According to the following proportions: mix 1 mmol of epothilone B, 3.5 mmol of methacrylic acid, 30 mmol of ethylene glycol dimethacrylate, 5 mg of azobisisobutylcyanide, and 3 ml of acetonitrile into the reaction tube, pass nitrogen and oxygen for 30 minutes, Under the protection of nitrogen, the reaction tube was sealed, placed in a water bath at 50°C for 24 hours, and then polymerized in a water bath at 60°C for 12 hours to obtain a polymerization reaction product;
[0068] Crushing, grinding, and sieving the obtained reaction product to obtain polymer particles with a particle size of 40-60 μm;
[0069] After the obtained polymer particles were washed with water, they were extracted with a Soxhlet extractor in a mixed solution of acetic acid:methanol (volume ratio 3:7) for 48 hours, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com