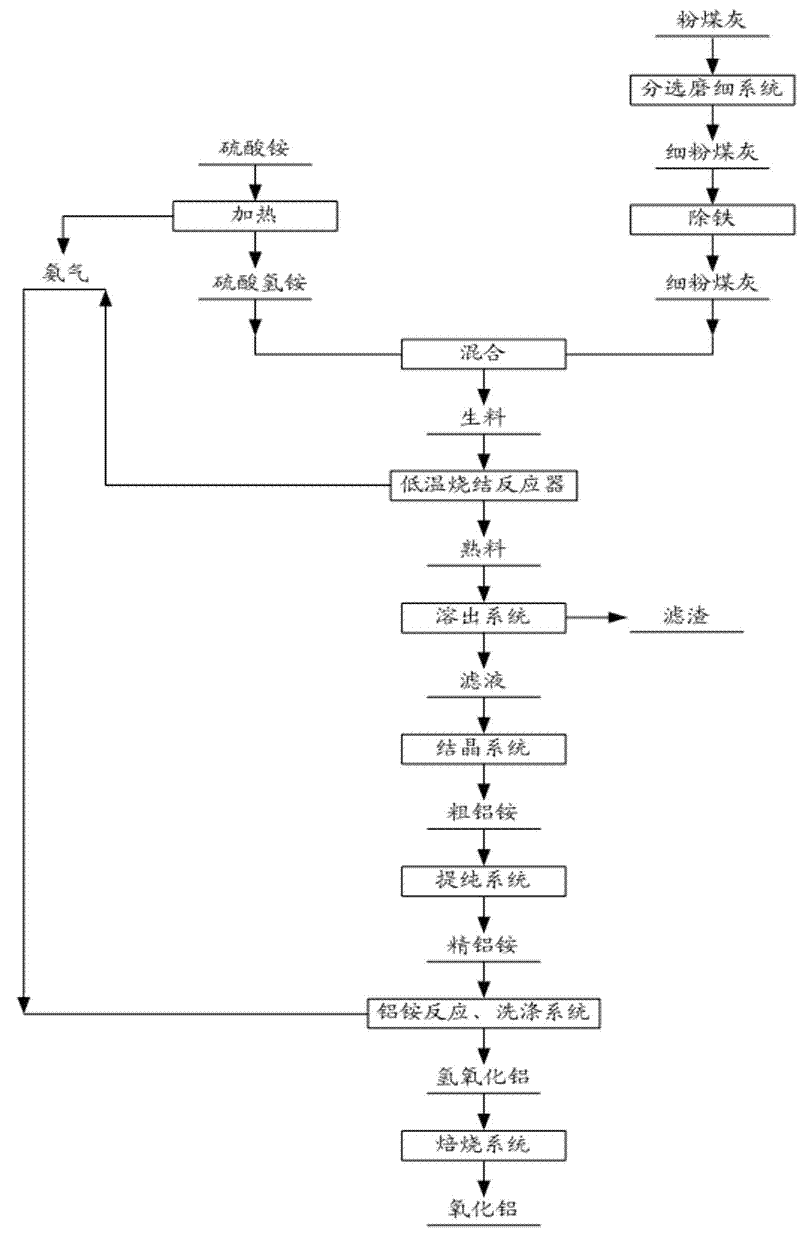
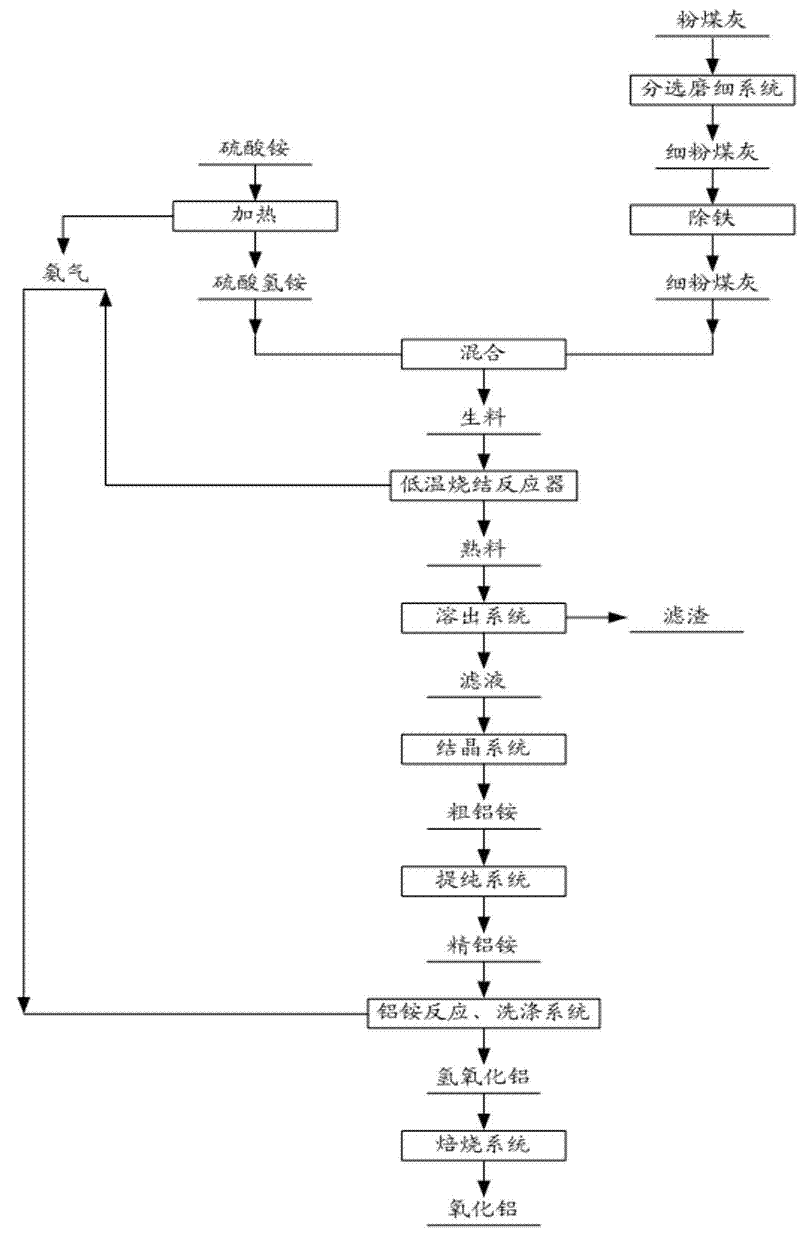
Method for extracting aluminium oxide from pulverized fuel ash
A technology of fly ash and alumina, applied in the direction of alumina/hydroxide preparation, etc., can solve the problems of limited production scale, high equipment investment, long process flow, etc., and achieve a small amount of residue, simple method, and convenient reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Grind fly ash containing 46% alumina and 40% silica to 325 meshes, and at the same time, sinter ammonium sulfate with a mass of 1350g at 280°C for 3 hours to prepare liquid ammonium bisulfate, and The obtained ammonia gas is recovered with water to prepare ammonia water.
[0053] According to the mass ratio of ammonium bisulfate / alumina in fly ash is 6.0, weigh the pulverized fly ash and ammonium bisulfate for sintering. The sintering temperature is 450°C, and the sintering time is 3.5 hours. It was dissolved at 98°C under normal pressure, and the dissolution rate of aluminum oxide in the ammonium aluminum sulfate solution obtained was 90%. The obtained aluminum ammonium sulfate crystals are dissolved and prepared into a 0.2mol / L aluminum ammonium sulfate solution, and then ammonia gas is introduced to decompose it, and the NH 3 The molar ratio to ammonium aluminum sulfate is 3.1, the reaction temperature is 35°C, and after 3 hours of reaction, an aluminum hydroxide so...
Embodiment 2
[0055] Grind fly ash containing 42% alumina and 40% silica to 200 meshes, and at the same time sinter ammonium sulfate with a mass of 2300g at 300°C for 2.5 hours to prepare liquid ammonium bisulfate. And recover the obtained ammonia gas with water to prepare ammonia water.
[0056] According to the mass ratio of ammonium bisulfate / alumina in fly ash is 6.5, weigh ammonium bisulfate and fly ash for sintering. The sintering temperature is 440°C and the sintering time is 2.5 hours. Dissolution at 98°C, the dissolution rate of aluminum oxide in the ammonium aluminum sulfate solution obtained was 89%. After removing impurities such as iron from the ammonium sulfate solution, the ammonium aluminum sulfate solution was cooled to 10°C to crystallize for 5 hours, and then the obtained ammonium aluminum sulfate The crystals were dissolved and prepared into 0.25mol / L aluminum ammonium sulfate solution, and then reacted with ammonia water with a concentration of 25%, NH 3 The molar rati...
Embodiment 3
[0058] Grind fly ash containing 40% alumina and 43% silica to 200 meshes, and at the same time, sinter 2000g of ammonium sulfate at 280°C for 3 hours to prepare liquid ammonium bisulfate, and The obtained ammonia gas is recovered with water to prepare ammonia water.
[0059] According to the mass ratio of ammonium bisulfate / alumina in fly ash to 7.2, weigh ammonium sulfate and fly ash for sintering. The sintering temperature is 320°C and the sintering time is 3.0 hours. ℃, the dissolution rate of aluminum oxide in the obtained ammonium aluminum sulfate solution was 92%. After removing impurities such as iron from the ammonium sulfate solution, the ammonium aluminum sulfate solution was evaporated and crystallized. The obtained aluminum ammonium sulfate crystals are dissolved and prepared into a 0.19mol / L aluminum ammonium sulfate solution, which is fed into ammonia gas for reaction, NH 3 The molar ratio to aluminum ammonium sulfate is 3.2, the reaction temperature is controll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com