Double-functional lithium battery electrolyte additive and preparation method thereof
An electrolyte additive, lithium battery technology, applied in secondary batteries, chemical instruments and methods, circuits, etc., can solve problems such as poor compatibility, negative electrode compatibility needs to be improved, etc., to achieve good wettability, low synthesis cost, and improved The effect of compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] DMAP synthesis method
[0047] Add 50mL of dry toluene into a 250mL three-necked flask, then add 12.4g (100mmol) of trimethyl phosphite, stir evenly, add 14.5g (100mmol) of 3-bromopropene, heat and reflux overnight, remove the solvent toluene, and then Distilled under reduced pressure to obtain 12.3 g of pure product with a yield of 82%. Using ESI-MS, 1 H NMR, 13 C NMR and 31 The product was characterized by P NMR, and the result confirmed that it was the target product. Data are as follows:
[0048] 1 H NMR (CDCl 3 , 400MHz): δ5.77-5.65(m, 1H), 5.20-5.11(m, 2H), 3.67(d, J=10.8Hz, 6H), 2.55(ddt, J H-P =22.0Hz, J=7.4Hz, J=1.3Hz, 2H). 13 C NMR (CDCl 3 , 100MHz): δ127.1(d, J C-P =11.3Hz), 120.1(d, J C-P =14.4Hz), 52.6(d, J C-P =6.7Hz), 31.2(d, J C-P =139.5Hz). 31 P NMR (CDCl 3 , 162MHz): δ28.66.ESI-MS: m / z=151[M+H] + ;173[M+Na] + .
Embodiment 2
[0050] DEAP synthesis method
[0051] After mixing 6.66g (55mmol) of 3-bromopropene and 8.3g (50mmol) of triethyl phosphite, the oil bath was heated to 160°C, reacted overnight, and distilled under reduced pressure to obtain 8.2g of pure product with a yield of 92%. The product was characterized by ESI-MS, 1H NMR, 13C NMR and 31P NMR, and the results confirmed that it was the target product. Data are as follows:
[0052] 1 H NMR (CDCl 3 , 400MHz): δ5.82-5.70(m, 1H), 5.22-5.13(m, 2H), 4.12-4.01(m, 4H)), 2.58(ddt, J H-P =22.0Hz, J=7.4Hz, J=1.3Hz, 2H), 1.28(t, J=7.1Hz, 6H). 13 C NMR (CDCl 3 , 100MHz): δ127.5(d, J C-P = 11.3Hz), 119.9(d, J C-P =14.5Hz), 61.8(d, J C-P =6.6Hz), 31.7(d, J C-P =139.4Hz), 16.4(d,J C-P =6.0Hz). 31 P NMR (CDCl 3 , 162MHz): δ29.67.ESI-MS: m / z=179[M+H] + ;201[M+Na] + .
[0053] The alkenyl phosphate compounds prepared in Examples 1 and 2 can be directly used as flame retardant additives or co-solvents for lithium-ion battery electrolytes.
Embodiment 3
[0055] The alkenyl phosphate flame retardant in this example is a phosphorus-containing organic compound with the following structural formula:
[0056]
[0057] That is, R1 and R2 in the general formula are methyl, R3 is allyl, and the above compound is named Dimethyl acrylicphosphonate, DMAP for short.
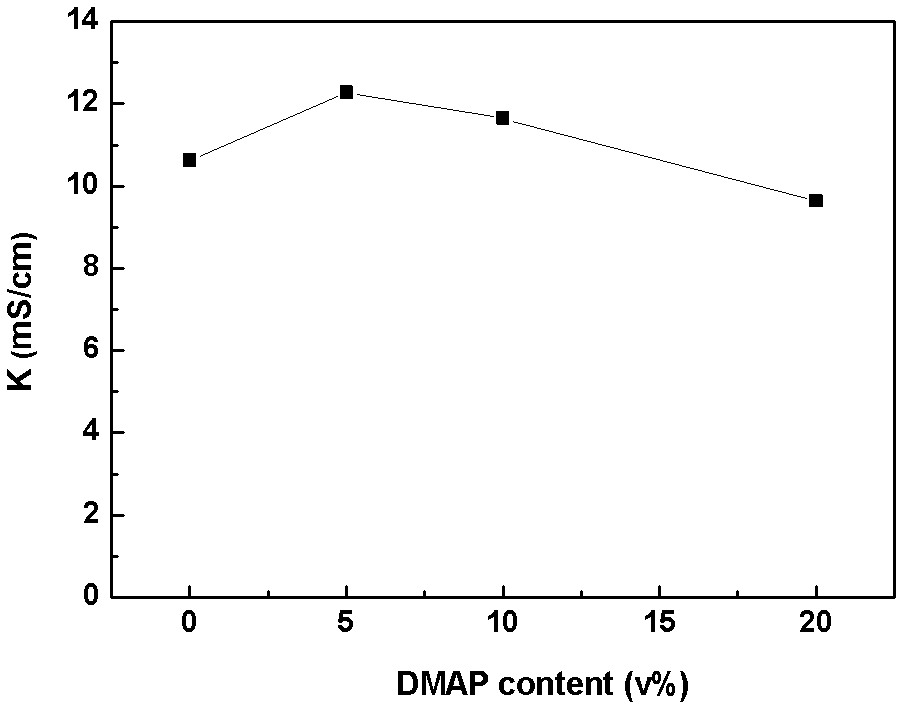
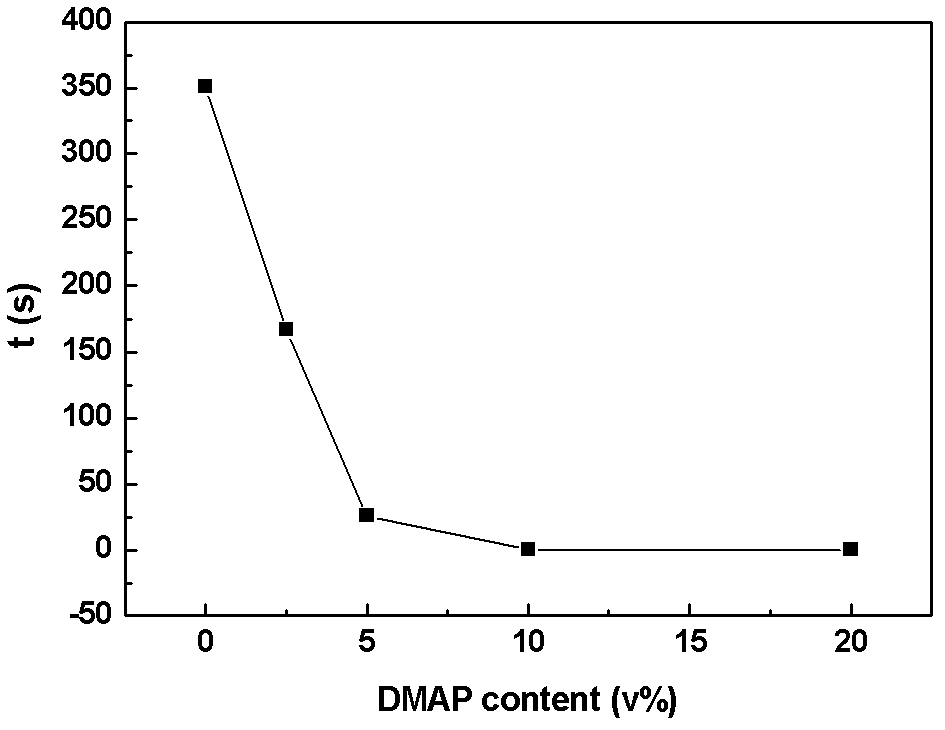
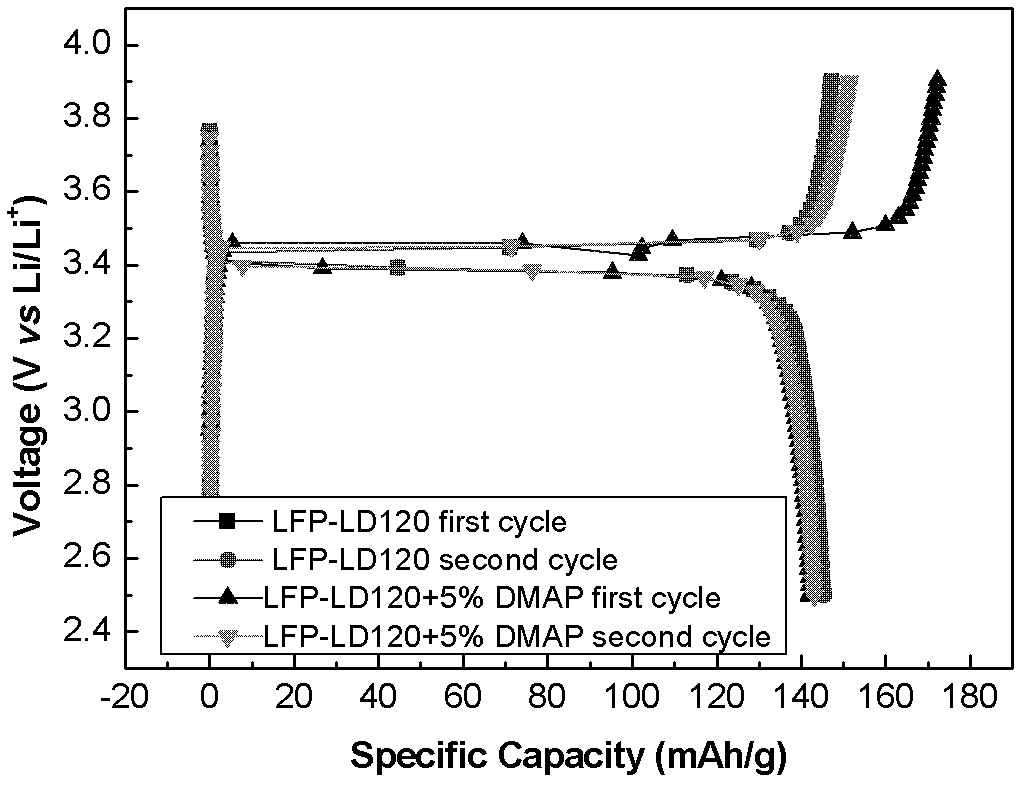
[0058] The above-mentioned alkenyl-containing phosphate is added to a commercial lithium-ion battery electrolyte, and the composition of the commercial lithium-ion battery electrolyte is 1 mol / L LiPF6 EC:DMC (50:50, v:v%) solution. The contents of the flame retardant are 2.5v%, 5v%, 10v%, and 20v%, and an appropriate amount of LiPF6 is added to the electrolyte so that the LiPF6 concentration of the electrolyte is still 1mol / L, and four kinds of electrolytes containing flame retardants are obtained , respectively named 2.5% DMAP, 5% DMAP, 10% DMAP, 20% DMAP.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com