Chlorobenzene derivative, optically isotropic liquid crystal medium, and optical element
A liquid crystal compound and compound technology, which is applied in optics, liquid crystal materials, nonlinear optics, etc., can solve problems such as unrecognizable compound properties, reduced operating voltage, and high voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0322] The resulting compound is obtained by 1 The nuclear magnetic resonance spectrum obtained in the H-NMR analysis, the gas chromatogram obtained in the gas chromatography (gas chromatography, GC) analysis, etc. are identified, and therefore the analysis method will be described first.
[0323] 1 H-NMR analysis: DRX-500 (manufactured by Bruker BioSpin Co., Ltd.) was used as a measurement device. Dissolve the samples prepared in Examples etc. in CDCl 3 In the deuterated solvent in which the sample is soluble, the measurement is carried out at room temperature, 500 MHz, and the cumulative number of times is 24. In addition, in the description of the obtained nuclear magnetic resonance spectrum, s represents a singlet, d represents a doublet, t represents a triplet, q represents a quartet, and m represents a multiplet. Also, tetramethylsilane (TMS) was used as a zero point standard substance for the chemical shift δ value.
[0324] GC analysis: A GC-14B gas chromatograph m...
example 1
[0352] Synthesis of compound (S1-11)
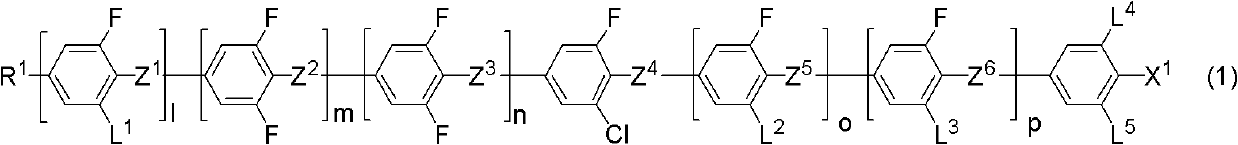
[0353] Compound (S1-11) is in formula (1-4-3), R 1 for C 4 h 9 , L 1 is hydrogen, L 2 , L 4 and L 5 Both are fluorine, X 1 for-CF 3 compound of. (same as compound (1-4-3-a))
[0354]
[0355] The synthesis process is shown in the figure below.
[0356]
[0357] where r.t. is room temperature.
[0358] Synthesis of compound (S1-2)
[0359] Under a nitrogen atmosphere, prepare a Grignard reagent from 11.3 g of dry magnesium and 75.0 g of 1-bromo-3-chloro-5-fluorobenzene (S1-1) using 220 ml of tetrahydrofuran (hereinafter referred to as THF), and cool it to -70°C. A solution of 52.0 g of trimethyl borate in 200 ml of THF was added dropwise there, and after stirring at this temperature for 3 hours, it was heated to room temperature over 1 hour and stirred for 12 hours. 2N-hydrochloric acid was added dropwise to the reaction solution and stirred for 1 hour, the product was extracted with eth...
example 2
[0383] Synthesis of compound (S2-1)
[0384] Compound (S2-1) is in formula (1-4-3), R 1 for C 5 h 11 , L 1 is hydrogen, L 2 , L 4 and L 5 Both are fluorine, X 1 for-CF 3 compound of.
[0385]
[0386] Synthesis of compound (S2-1)
[0387] The synthesis of (S2-1) was carried out according to the synthesis method of (S1-11) in Example 1. The phase transition temperature of the obtained compound (S2-1) is shown below.
[0388] Phase transition temperature (°C): K 91.4 (N 34.4)I.
[0389] 1 The chemical shift δ (ppm) of the H-NMR analysis is shown below, and the obtained compound was identified as (S2-1). In addition, the determination solvent is CDCl 3 . Chemical shift δ(ppm): 7.53(m, 1H), 7.37~7.33(m, 2H), 7.12~7.07(m, 3H), 7.04~7.01(m, 3H), 2.66(t, 2H), 1.69~ 1.63(m, 2H), 1.41~1.31(m, 4H), 0.92(t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com