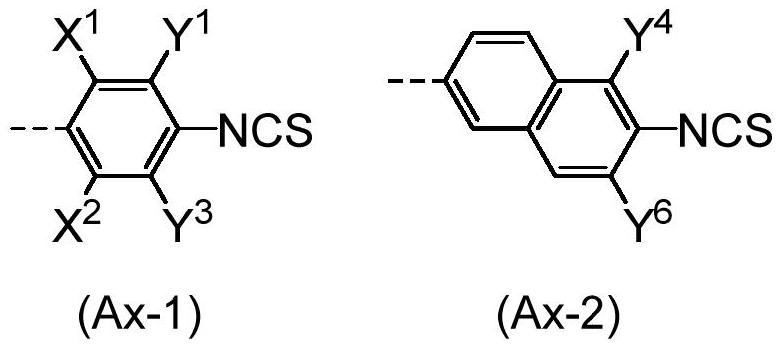
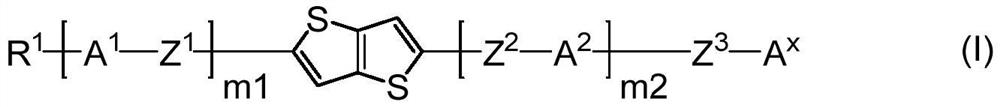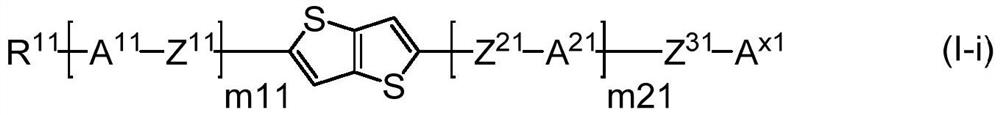Compound, composition, liquid crystal composition, and high-frequency phase shifter
A compound and independent technology, applied in liquid crystal materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of low compatibility, insufficient phase modulation characteristics, low dielectric constant anisotropy, etc., and achieve compatibility The effect of high and large dielectric constant anisotropy and large refractive index anisotropy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0322] (Example 1) Production of a compound represented by formula (I-1)
[0323] [chem 63]
[0324]
[0325] [chem 64]
[0326]
[0327] 7.0 g of the compound represented by the formula (I-1-1) and 70 mL of dichloromethane were added to the reaction container. 9.3 g of N-bromosuccinimide was added little by little while cooling with ice, and stirred at room temperature for 5 hours. The reaction liquid was poured into water, and a liquid separation process was performed. The organic layer was washed with brine, and purified by column chromatography (silica gel, dichloromethane / hexane) to obtain 8.8 g of a compound represented by the formula (I-1-2).
[0328] Under nitrogen atmosphere, in reaction container, add the compound represented by 8.8g formula (I-1-2), 8.3g potassium carbonate, the compound represented by 8.6g formula (I-1-3), 88mL toluene, 44mL ethanol, 44 mL of water, 0.3 g of [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) dichloromethane adduc...
Embodiment 2
[0338] (Example 2) Production of a compound represented by formula (I-2)
[0339] [chem 65]
[0340]
[0341]7.0 g of the compound represented by the formula (I-2-1) and 70 mL of dichloromethane were added to the reaction vessel. 7.9 g of N-iodosuccinimide was added little by little while cooling with ice, and stirred at room temperature for 5 hours. The reaction liquid was poured into water, and a liquid separation process was performed. The organic layer was washed with saline, and purified by column chromatography (silica gel, dichloromethane / hexane) to obtain 8.8 g of a compound represented by the formula (I-2-2).
[0342] Under a nitrogen atmosphere, add 8.8g of the compound represented by the formula (I-2-2), 0.2g of copper iodide (I), 0.2g of 2-dicyclohexylphosphino-2',4',6 '-triisopropylbiphenyl [XPhos], 0.2 g palladium(II) acetate, 88 mL diisopropylamine, 176 mL N,N-dimethylformamide. A solution obtained by dissolving 5.4 g of the compound represented by the fo...
Embodiment 3
[0348] (Example 3) Production of the compound represented by formula (I-3)
[0349] [chem 66]
[0350]
[0351] [chem 67]
[0352]
[0353] Under nitrogen atmosphere, 7.0 g of the compound represented by the formula (I-3-1), 4.3 g of pyridine, and 70 mL of dichloromethane were added to the reaction vessel. 11.3 g of trifluoromethanesulfonic anhydride was added dropwise while cooling with ice, and stirred at room temperature for 4 hours. The reaction solution was poured into a 5% aqueous solution of sodium bicarbonate for liquid separation. The organic layer was washed sequentially with 5% hydrochloric acid, water and brine, and then purified by column chromatography (silica gel, dichloromethane / hexane) to obtain 10.6 g of a compound represented by the formula (I-3-2).
[0354] Under nitrogen atmosphere, add the compound represented by 10.6g formula (I-3-2), 9.6g potassium acetate, 10.0g bis(pinacolate) diboron, 106mL dimethyl sulfoxide, 0.4g [1,1'-bis(diphenylphosphi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com