Method for synthesizing decyl acetal aldehyde
A technology for the synthesis of ten-carbon acetal and its synthesis method, which is applied in the field of synthesis of ten-carbon acetal, can solve the problems of numerous steps, high cost, and low yield, and achieve simple industrial operation, good industrialization prospects, and technical difficulty little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] A kind of synthetic method of ten carbon acetals, comprises the following steps:
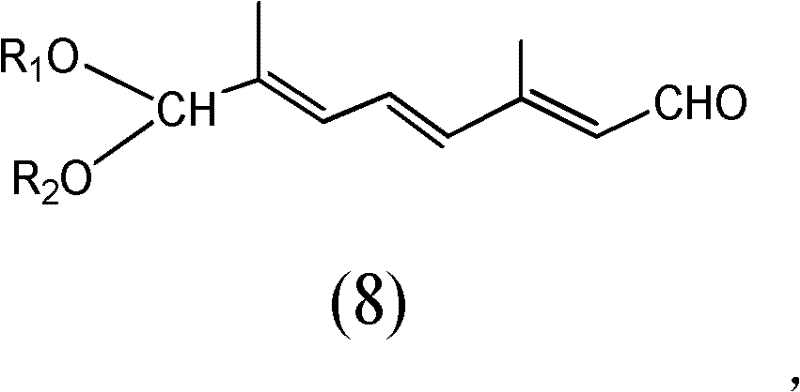
[0026] 1) Compound (E-4-acetoxy-2-methyl-2-butene-1-aldehyde) represented by structural formula (1) and trimethyl orthoformate or triethyl orthoformate at room temperature, acidic Carry out acetalization reaction under the effect of catalyzer p-toluenesulfonic acid, add alkali to carry out hydrolysis again after reaction finishes, obtain the acetal product as shown in structural formula (2),
[0027]
[0028] Among them, R 1 It is methyl or ethyl, and the molar ratio of the compound shown in structural formula (1), trimethyl orthoformate or triethyl orthoformate, and base is 1: 0.8-1.5: 0.6-1.2;
[0029] 2) the acetal product obtained in the previous step and the vinyl ether compound shown in (3) according to the molar ratio of 1: 0.8-1.2 at room temperature, catalyst (zinc chloride, zinc bromide, trichloride A kind of in iron) reacts under the effect, obtains the product of structur...
Embodiment 1
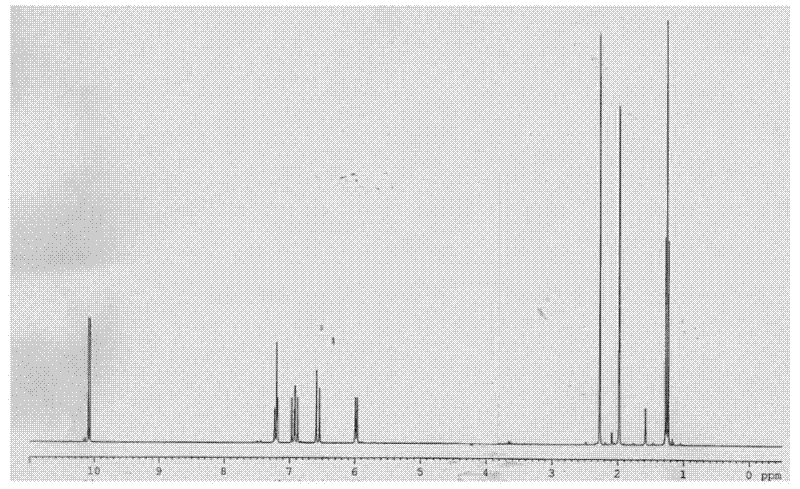
[0041] The preparation of the compound shown in structural formula as (8)
[0042]
[0043] Get 10 grams of the compound shown in structural formula (1), add catalytic amount of p-toluenesulfonic acid, add 10 grams of trimethyl orthoformate, react at room temperature for 10 hours, add 2 grams of hydrogen after the reaction finishes Sodium oxide, stirred and reacted for 6 hours, then added 200 milliliters of water, extracted three times with dichloromethane, 50 milliliters each time, combined the organic phases, backwashed the organic layer with 5% saline, 50 milliliters each time, dried with potassium carbonate, Filter, evaporate the solvent to dryness, and distill under reduced pressure to obtain 9 grams of the reaction product, the GC content is 98%, and the reaction equation is shown as follows:
[0044]
[0045]
Embodiment 2
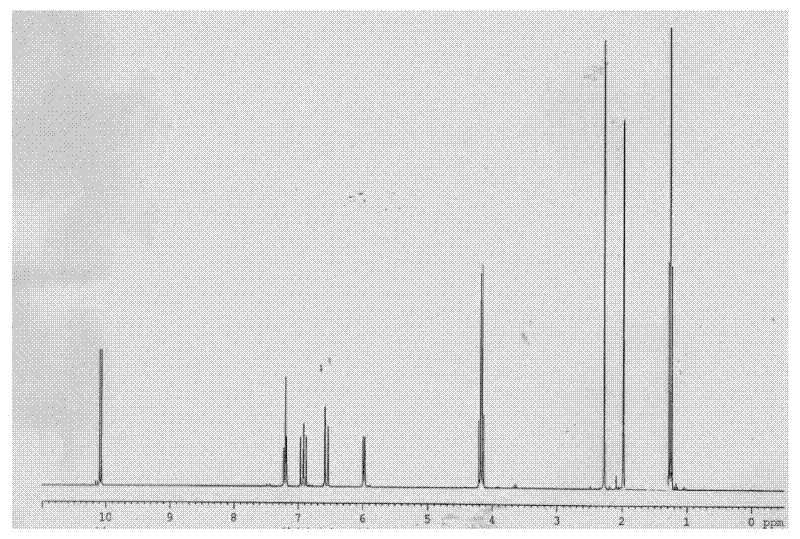
[0047] The preparation of the compound shown in structural formula as (9)
[0048]
[0049] Get 10 grams of the compound shown in structural formula (1), add catalytic amount of p-toluenesulfonic acid, add 11 grams of triethyl orthoformate, react at room temperature for 15 hours, add 2 grams of hydrogen after the reaction finishes Sodium oxide, stirred and reacted for 7 hours, then added 200 milliliters of water, extracted three times with dichloromethane, 50 milliliters each, combined the organic phases, backwashed the organic layer with 5% saline, 50 milliliters each, dried with potassium carbonate, Filter, evaporate the solvent to dryness, and distill under reduced pressure to obtain 10 grams of the reaction product, the GC content is 97%, and the reaction equation is shown as follows:
[0050]
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com