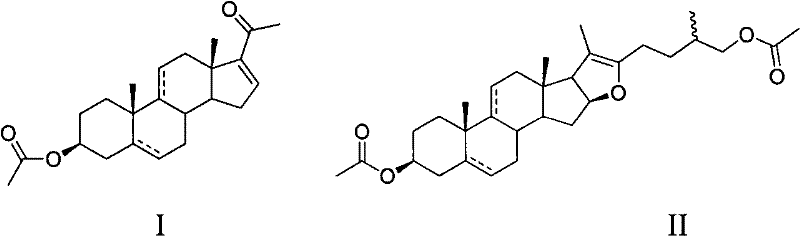
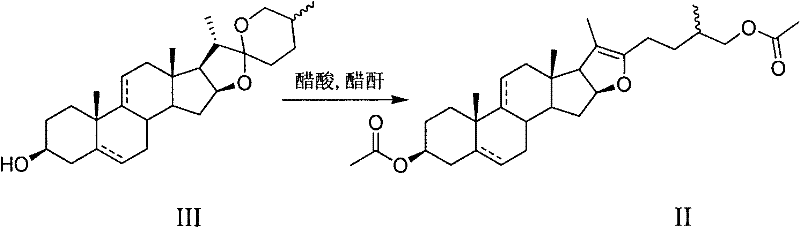
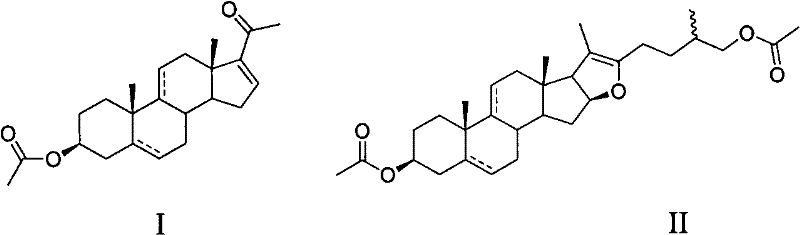
Method for synthetizing dehydropregnenolone acetate and isotype substance thereof
A kind of technology of gestational dienolone acetate and similar substances, applied in the field of steroid drug raw material manufacturing, can solve the problems of large consumption of organic solvent, complicated operation and the like, and achieve the effects of saving organic solvent and reducing production and labor costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Oxidation of pseudodiosgenin acetate to gestational dienolone acetate:
[0019] Put 50 g of diosgenin in a pressurized kettle, add acetic anhydride and acetic acid to dissolve, heat for 1 hour, recover acetic acid and acetic anhydride under reduced pressure, and recrystallize the obtained crude pseudodiosgenin acetate with methanol to obtain pseudodiosgenin B Pure esters.
[0020] In the reaction flask, add 10g (20mmol) pseudodiosgenin acetate and 25mL acetic acid, then add 0.07g (0.2mmol) Na(NH 4 ) 2 VO 4 .Na 2 NH 4 VO 4 , 4.56g (40mmol) hydrogen peroxide (30%H 2 o 2 ), the reaction was stirred at 5°C for 10 hours. Add 1.9g sodium acetate, react for 2 hours at 80°C, add water, precipitate out, crystallize with ethanol, and obtain 5.5g acetate dienolone by filtration, the product yield is 77%, m.p.165-167°C, proton nuclear magnetic resonance spectrum ( 400MHz, CDCl 3 )δ: 6.72(dd, J=2Hz, 1H, 16-H), 5.40(d, J=5.2Hz, 1H, 6-H), 4.62(m, 1H, 3-H), 2.02(s, 3H , 3-CH...
Embodiment 2
[0022] Oxidation of sisal-genin acetate to 3β-acetoxy-5α-pregna-16(17)-en-20-one:
[0023] Put 50g of sisal sapogenin in a pressurized kettle, add acetic anhydride and acetic acid to dissolve, heat for 1 hour, recover acetic acid and acetic anhydride under reduced pressure, and recrystallize the obtained crude pseudo sisal sapogenin acetate with methanol to obtain pseudo sisal Pure saponin acetate.
[0024] In the reaction flask, add 10g (20mmol) pseudosisalin acetate and 15mL acetic acid, then add 0.15g (0.8mmol) V 2 o 5 , 22.8g (200mmol) hydrogen peroxide (30%H 2 o 2 ), stirred and reacted at 40° C. for 2 hours. Add 1.9g of sodium acetate, react at 110°C for 5 hours, add water, precipitate out, crystallize with ethanol, and obtain 5.6g of 3β-acetoxy-5α-pregna-16(17)-en-20-one by filtration. The rate is 78%. m.p.162-165℃, hydrogen nuclear magnetic resonance spectrum (400MHz, CDCl 3 )δ: 6.70(dd, J=2Hz, 1H, 16-H), 4.70(m, 1H, 3-H), 2.04(s, 3H, 3-CH 3 COO), 2.27 (s, 3H, ...
Embodiment 3
[0026] Oxidation of pseudo-9(11)-deshydrosisalin acetate to 3β-acetoxy-5α-pregna-9(11),16(17)-dien-20-one:
[0027] In the reaction flask, add 10g (20mmol) pseudo-9(11)-dehydrosisalin acetate and 35mL acetic acid, then add 69.6g (200mmol) vanadium acetylacetonate, 5.7g (50mmol) hydrogen peroxide (30%H 2 o 2 ), stirred and reacted at 40° C. for 2 hours. Add 1.9g of sodium acetate, react at 110°C for 0.5 hours, add water, precipitate out, crystallize with ethanol, and filter to obtain 6.5g of 3β-acetoxy-5α-pregna-9(11), 16(17)-diene- 20-keto. Yield 91%. m.p.165-189℃, hydrogen nuclear magnetic resonance spectrum (400MHz, CDCl 3 )δ: 6.72(dd, J=2Hz, 1H, 16-H), 5.37(d, J=5.6Hz, 1H, 11-H), 4.69(m, 1H, 3-H), 2.04(s, 3H , 3-CH 3 COO-), 2.29 (s, 3H, 21-H), 0.82 (s, 3H, 18-H), 0.87 (s, 3H, 19-H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com