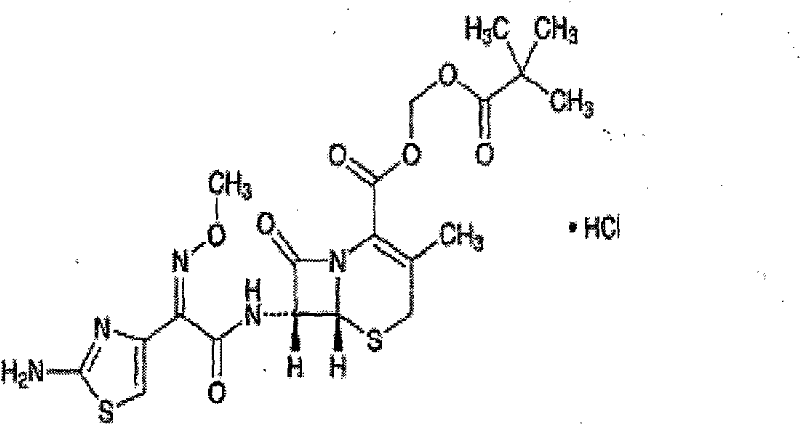
Cefetamet pivoxil hydrochloride liposome solid preparation
A technology of ceftamet pivoxil hydrochloride and solid preparations, which is applied in the field of ceftamet pivoxil hydrochloride liposome solid preparations and its preparation method, can solve the problems of low bioavailability, unsatisfactory long-term stability, and Encapsulation efficiency is not high, to achieve the effect of improving dissolution and bioavailability, significant curative effect, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
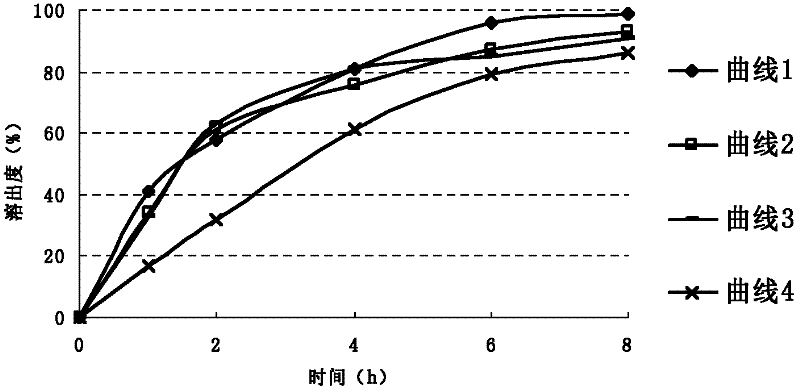
Image
Examples
Embodiment 1
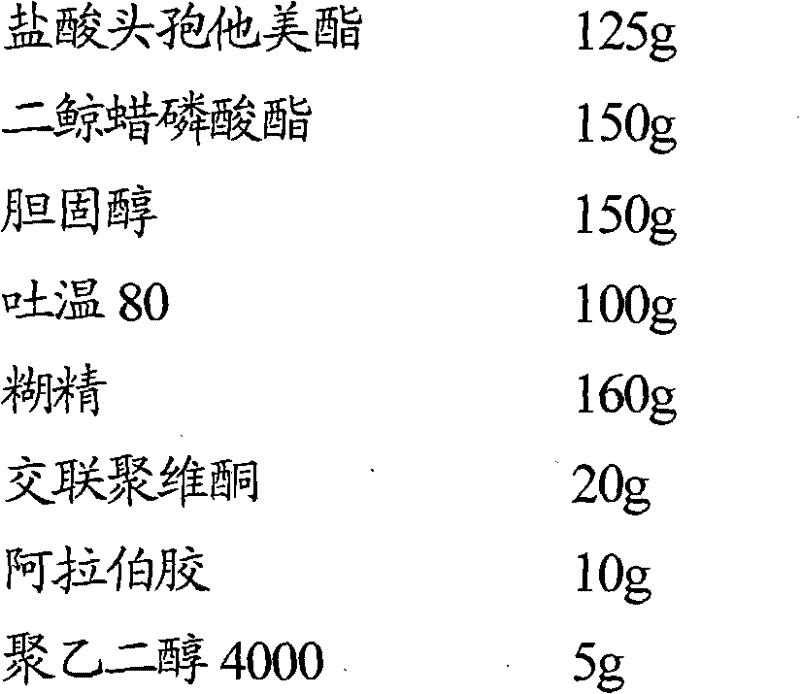
[0043] Embodiment 1 Ceftamet pivoxil hydrochloride liposome tablet
[0044] Prescription (1000 tablets)
[0045]
[0046] Preparation Process:
[0047] (1) Accurately weigh 150g of dicetyl phosphate, 150g of cholesterol, and 100g of Tween 80, place in a pear-shaped bottle, add 1000ml of isopropanol, mix well, place in a rotary evaporator and evaporate to dryness for 20 minutes to form a film;
[0048] (2) Adding 125g ceftazidime hydrochloride and 1000ml pH in the pear-shaped bottle is a phosphate buffered saline solution of 6.2, spins and dissolves for 30 minutes, ultrasonically makes the solution transparent for 15 minutes, and filters through a 0.45 μm microporous membrane;
[0049] (3) The above-mentioned filtrate is placed in -20 ℃ and frozen for 5 hours, taken out, placed at room temperature to melt, then ultrasonicated for 5 minutes, and spray-dried to obtain ceftazidime hydrochloride liposome powder;
[0050] (4) Mix ceftazidime hydrochloride liposome powder with 1...
Embodiment 2
[0053] Embodiment 2 Cefetamet pivoxil hydrochloride liposome sheet
[0054] Prescription (1000 tablets)
[0055]
[0056] Preparation Process:
[0057] (1) Weigh 100g dicetyl phosphate, 200g cholesterol, 150g Tween 80, place in a pear-shaped bottle, add 1000ml isopropanol, mix well, place in a rotary evaporator and evaporate to dryness for 60 minutes to form a film;
[0058] (2) Adding 250g ceftazidime hydrochloride and 1000ml pH in the pear-shaped bottle is a phosphate buffered saline solution of 6.2, spins and dissolves for 30 minutes, ultrasonically makes the solution transparent for 30 minutes, and filters with a 0.45 μm microporous membrane;
[0059] (3) The above-mentioned filtrate is placed in -20 ℃ and frozen for 12 hours, taken out, melted, and then ultrasonicated for 10 minutes, and spray-dried to obtain ceftazidime hydrochloride liposome powder;
[0060] (4) Ceftamet pivoxil hydrochloride liposome powder is mixed with 200g dextrin, 15g crospovidone, crosses 80 ...
Embodiment 3
[0063] Embodiment 3 Cefetamet pivoxil hydrochloride liposome particle
[0064] Prescription (1000 bags)
[0065]
[0066]
[0067] Preparation Process:
[0068] (1) Weigh 180g dicetyl phosphate, 160g cholesterol, 50g Tween 80, place in a pear-shaped bottle, add 1000ml chloroform, place in a rotary evaporator and evaporate to dryness for 120 minutes to form a film;
[0069] (2) Add 250g ceftazidime hydrochloride and 1000ml pH to the citrate buffer of 6.2 in the pear-shaped bottle, rotate and dissolve for 30 minutes, ultrasonically make the solution transparent for 30 minutes, and filter through a 0.45 μm microporous membrane;
[0070] (3) The above-mentioned filtrate is placed in -20 DEG C and frozen for 5 hours, taken out, melted, and then ultrasonicated for 10 minutes, and spray-dried to obtain ceftazidime hydrochloride liposome powder;
[0071] (4) Mix ceftazidime pivoxil hydrochloride liposome powder with 240g lactose, 20g crospovidone and 1g steviol glycoside, cros...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com