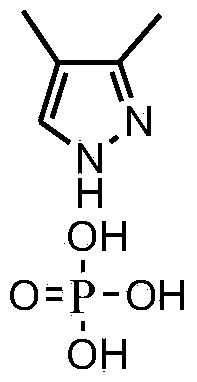
Synthesizing method of 3,4-dimethylpyrazole phosphate (DMPP)
A technology of dimethylpyrazole phosphate and dimethylpyrazole is applied in the field of synthesis of 3,4-dimethylpyrazole phosphate, and can solve the problem of high price of starting materials, many by-products, and high yield. Low problems, to achieve the effect of cheap raw materials, less environmental pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 144g (2.0mol) butanone, 90g (3.0mol) paraformaldehyde, and 5g methanol to the reaction flask, heat up to 40-50°C, add 3g, 10% potassium hydroxide solution dropwise, after the dropwise addition is completed, keep the same temperature After reacting for 15 hours, add 145g (2.3mol) hydrazine hydrate, 200g toluene, 0.5g p-toluenesulfonic acid, heat up and reflux for dehydration reaction, separate the generated water during the reaction until no water is generated, react for 10 hours, and then Add this reaction solution to 1120g, 70% (8.0mol) sulfuric acid solution containing 2g sodium iodide and 0.1g tetrabutylammonium bromide, raise the temperature to 80-90°C and react for 12h, the gas phase detection reaction is complete, and cool down to 30 ℃, use 30% sodium hydroxide solution to adjust the pH to 9, add 200g toluene, separate layers, take the organic phase, and distill off the solvent to obtain 3,4-dimethylpyrazole, 3,4-dimethylpyrazole Dissolve it in 500g of ethanol...
Embodiment 2
[0024] Add 144g (2.0mol) butanone, 90g (3.0mol) paraformaldehyde, and 5g methanol to the reaction flask, heat up to 40-50°C, add 3g, 10% sodium hydroxide solution dropwise, after the dropwise addition, keep the same temperature After reacting for 15 hours, add 145g (2.3mol) hydrazine hydrate, 200g toluene, 0.5g p-toluenesulfonic acid, heat up and reflux for dehydration reaction, separate the generated water during the reaction until no water is generated, react for 10 hours, and then Add this reaction solution to 1120g, 70% (8.0mol) sulfuric acid solution containing 2g sodium iodide and 0.1g tetrabutylammonium bromide, raise the temperature to 70-80°C and react for 14h, the gas phase detection reaction is complete, and cool down to 30 ℃, use 30% sodium hydroxide solution to adjust the pH to 9, add 200g toluene, separate layers, take the organic phase, and distill off the solvent to obtain 3,4-dimethylpyrazole, 3,4-dimethylpyrazole Dissolve it in 500g of ethanol, add phosphoric...
Embodiment 3
[0026]Add 144g (2.0mol) butanone, 90g (3.0mol) paraformaldehyde, and 5g methanol to the reaction flask, heat up to 40-50°C, add 3g, 10% potassium hydroxide solution dropwise, after the dropwise addition is completed, keep the same temperature After reacting for 15 hours, add 145g (2.3mol) hydrazine hydrate, 200g toluene, 0.5g p-toluenesulfonic acid, heat up and reflux for dehydration reaction, separate the generated water during the reaction until no water is generated, react for 10 hours, and then Add this reaction solution to 1120g, 70% (8.0mol) sulfuric acid solution containing 2g sodium iodide and 0.1g tetrabutylammonium bromide, raise the temperature to 80-90°C and react for 12h, the gas phase detection reaction is complete, and cool down to 30 ℃, use 30% sodium hydroxide solution to adjust the pH to 9, add 200g toluene, separate layers, take the organic phase, and distill off the solvent to obtain 3,4-dimethylpyrazole, 3,4-dimethylpyrazole Dissolve in 500g of methanol, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com