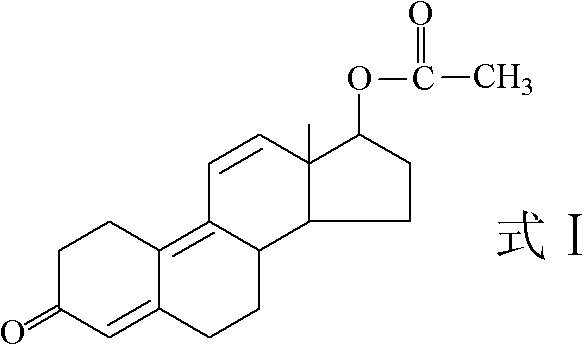
Preparation method for trenbolone acetic ester
A technology of dragon acetate and temporolone, which is applied in the field of preparation of steroid compound tambolone acetate, can solve problems such as inability to obtain solids, and achieves the effects of short reaction route, high yield and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
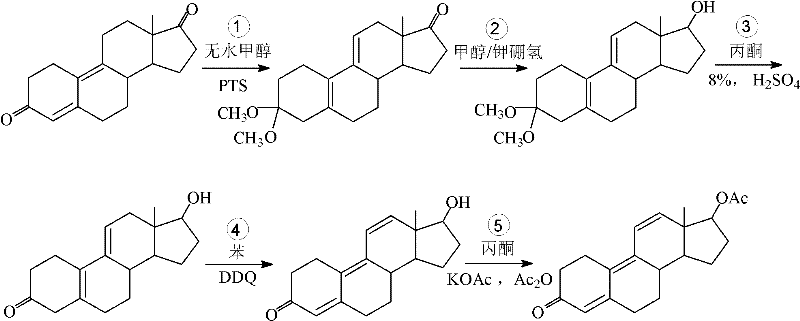
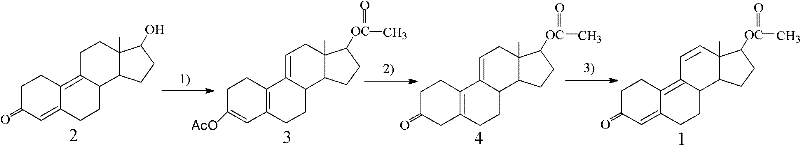
[0044] In order to solve the shortcomings of the existing method for preparing tambolone acetate, such as long synthetic route, complicated operation and low yield, the present invention provides a short reaction route, high reaction selectivity, less by-products and high yield. Preparation method of tambolone acetate. The method is based on 17β-hydroxyl-estro-4,9-dien-3-one as a starting material (compound 2), and comprises the following steps in turn:
[0045]
[0046] 1) Esterification of the 17β hydroxyl group of compound 2 and the esterification of the 3-position enol to obtain 5(10), 9(11)-estratriene-3, 17β-diol diacetate (compound 3);
[0047] 2) The 3-position enol ester of compound 3 was hydrolyzed to obtain 17β-hydroxy-estro-5(10), 9(11)-dien-3-one acetate (compound 4);
[0048] 3) The 5(10), 9(11) conjugated double bonds of compound 4 were dehydrogenated by dichlorodicyanoquinone (DDQ) to obtain tambolone acetate (compound 1).
[0049] The following examples a...
Embodiment 1
[0051] Embodiment 1, prepare tamburone acetate
[0052] Prepare tambolone acetate with the method of the present invention, and concrete method comprises the following steps:
[0053] 1) Obtaining of 3,5(10),9(11)-estratriene-3,17β-diol diacetate (compound 3)
[0054] Starting with 17β-hydroxy-estr-4,9-dien-3-one (compound 2, the starting material is 3-methoxy-estr-2,5(10)-diene- 17β-alcohol is used as a raw material, which can be prepared according to existing methods, such as the method reported in the literature (Wang Ruibin et al., Chinese Journal of Medicinal Chemistry, 1994, 4(3): 187-189), with a yield of 68.5% and a melting point of: 184-187℃, specific rotation: (C=1%, CHCl 3 ), content: 98.6% (HPLC); this raw material can also be obtained commercially, such as Beijing Ganxing Chemical Factory, etc.
[0055] Esterify its 17β-hydroxyl and simultaneously esterify the 3-position enol to obtain 3,5(10),9(11)-estratriene-3,17β-diol acetate (compound 3) The chemical rea...
Embodiment 2
[0074] Embodiment 2, preparation tempbolone acetate
[0075] Prepare tambolone acetate with the method of the present invention, and concrete method comprises the following steps:
[0076] 1) Obtaining of 3,5(10),9(11)-estratriene-3,17β-diol diacetate (compound 3)
[0077]Starting from 17β-hydroxy-estr-4,9-dien-3-one, esterify its 17-hydroxyl group and simultaneously esterify its 3-position enol to obtain 3,5(10),9(11)- Estratriene-3, the chemical reaction formula of 17β-diol diacetate (compound 3) is identical with embodiment 1, and concrete synthetic method is as follows:
[0078] A) Put acetic anhydride (600mL, 6.35mol), acetyl chloride (400mL, 5.61mol) and pyridine (10mL) into the reaction vessel in turn, put compound 2 (50g, 183.57mmol) under stirring, heat up to 80°C, and protect it under nitrogen keep the reaction for 1 hour;
[0079] B) When the heat preservation is over, cool the reaction solution to 20°C, slowly pour the reaction solution into 1200mL ice water whi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com