Chicken Marek's disease virus (MDV) miRNA (micro Ribonucleic Acid) deletion vaccine strain and application thereof
A chicken Marek's disease, gene deletion vaccine technology, applied in the field of biomedicine, can solve the problem that MD vaccine cannot provide effective protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
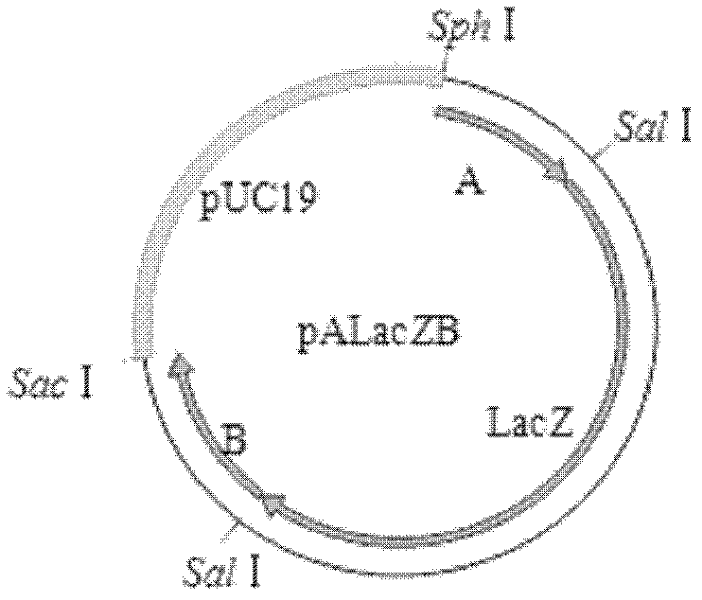
[0039] Embodiment 1 Construction of recombinant virus rMSΔmiR9-12 strain:
[0040] 1. Test materials: MDV MS strain (see literature: Construction of Marek's virus miRNA deletion strain and its growth in vitro, Yang Wenchuang, Liu Changjun, etc., Chinese Veterinary Science. 2011, 41 (03)), MDV Md5 (see literature : Marek virus super strain (Md5, RB1B) virulence test, Gan Junji, Liu Xiufan, Wu Changxin, Proceedings of the Eleventh Academic Symposium of the Poultry Disease Branch of the Chinese Society of Animal Husbandry and Veterinary Medicine. 2002), 814 strains of chicken Marek's disease virus (see Literature: Cloning and sequence analysis of gE, gI, gp82 genes of chicken Marek's disease virus 814 strains. Zhang Yanping, Liu Changjun, etc. Advances in Animal Science, 2007 (3)) Preserved and provided by the Laboratory of Avian Infectious Diseases, Harbin Veterinary Research Institute; no specific Pathogen (SPF) chicken embryos and SPF chickens were provided by the Animal Cente...
Embodiment 2
[0066] Example 2 Recombinant Virus Biological Characteristics and Immunoprotective Efficacy Analysis
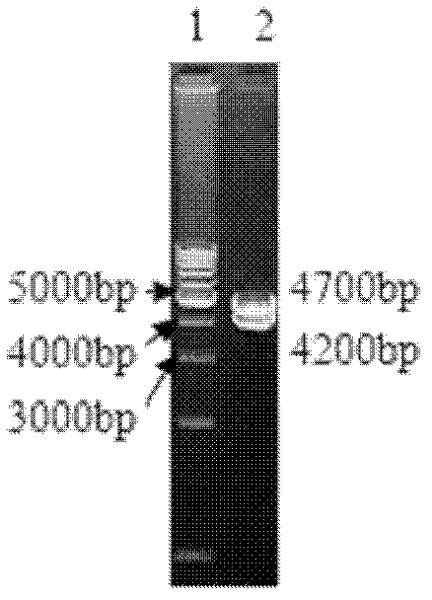
[0067] 1) Detection of the growth characteristics of the recombinant virus: draw the growth curve of the purified virus, and the specific steps are as follows: Dilute the MS strain and the recombinant virus rMSΔmiR9-12 to 100PFU / ml with 3% cell culture medium, and use a dose of 1ml per well Inoculate a 6-well plate of CEF primary cells that has been spread into a single layer. After the virus grows for 1, 2, 3, 4, 5, 6, and 7 days, the virus is digested with trypsin, and each virus is collected in 3 replicate wells per day. Extract the total DNA of the recombinant virus with the genomic DNA extraction kit of Tiangen Company, and measure the copy number (Copies / 106cells) per million cells of the virus with the method of double real-time fluorescent quantitative PCR, such as Figure 11 shown.
[0068] 2) Detection of genetic stability of recombinant virus: MS strain and recomb...
Embodiment 3
[0072] Embodiment 3 Recombinant Virus Immunoprotective Efficacy Analysis
[0073]160 1-day-old SPF white Laihang chickens were randomly divided into 6 groups, of which the first 5 groups had 30 chickens in each group, and the sixth group had 10 chickens. The first group was the recombinant virus immune group, and the second group was the recombinant immune super MDV Md5 challenged virus. The third group is the 814 virus immune group, the fourth group is the 814 virus immune super MDV Md5 challenge group, the fifth group is the super MDV Md5 direct challenge group, and the sixth group is the blank control group. The chicks were reared in a negative pressure isolator, and the chicks had free access to food and water. The immunization dose of recombinant virus rMSΔmiR9-12 and 814 virus was 2000PFU per mouse. On the 7th day after immunization, the challenge dose of supervirulent Md5 was 1000 PFU per mouse, intraperitoneally injected. From the date of inoculation, observe and rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com