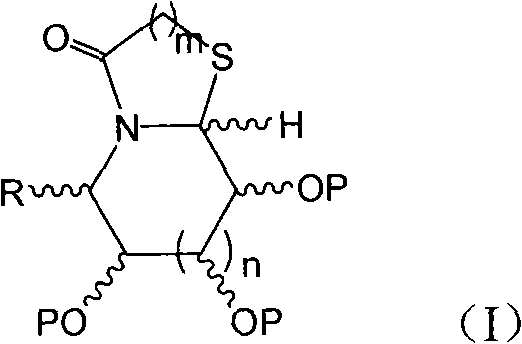
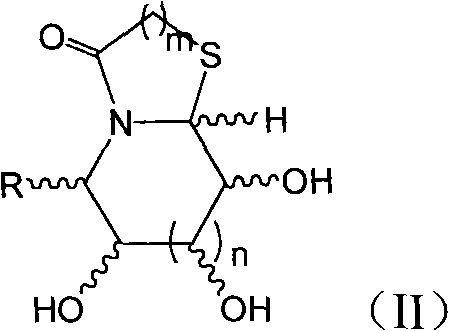
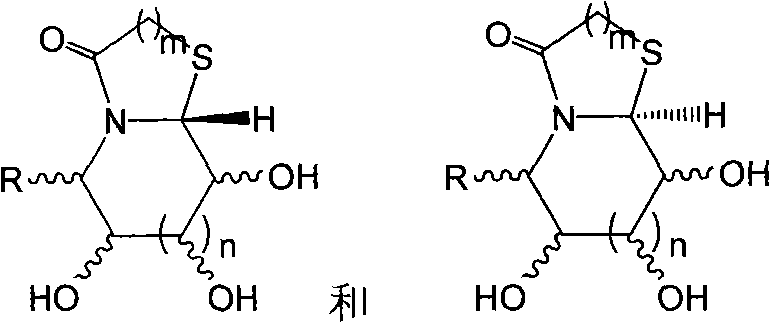
Thiazole (piperazine) azululanone azasugar derivative and synthesis method and application thereof to medicinal preparation
A technology of derivatives and azasaccharides, applied in the direction of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, antiviral agents, etc., to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Synthesis of 6R, 7R, 8R, 8aR)-tetrahydro-6-hydroxyl-7,8-O-isopropyl-2H-thiazo[3,2-a]pyridin-3(5H)-one (referred to as compound 2a) and (6R, 7R, 8R, 8aS)-tetrahydro-6-hydroxyl-7,8-O-isopropyl-2H-thiazo[3,2-a]pyridin-3(5H)-one (referred to as compound 3a )
[0060] Its chemical reaction formula is as follows:
[0061]
[0062] The specific method is:
[0063] In a 10mL microwave sealed tube, weigh 1mmol azido sugar 1a (1-hydroxy-2,3-O-isopropyl-5-azidofuran D ribose), and dissolve it in 3mL anhydrous toluene, add Triphenylphosphine 393mg (1.5mmol), microwave irradiation at 80°C for 5-8min., add thioglycolic acid 146μL (2.0mmol), continue microwave reaction at 80°C for 10-15min. After the reaction was completed, anhydrous potassium carbonate powder was added to neutralize the reaction solution to pH=7, and dichloromethane (3×10 mL) was added for extraction, the organic phases were combined, and silica gel column chromatography (ethyl acetate:cyclohexane 1:3-2:1), to...
Embodiment 2
[0079] Synthesis of (6R, 7S, 8S, 8aR)-tetrahydro-6-hydroxyl-7,8-O-isopropyl-2H-thiazo[3,2-a]pyridin-3(5H)-one (referred to as compound 2b ) and (6R, 7S, 8S, 8aS)-tetrahydro-6-hydroxyl-7,8-O-isopropyl-2H-thiazolo[3,2-a]pyridin-3(5H)-one (compound for short 3b)
[0080] Its chemical reaction formula is as follows:
[0081]
[0082] The specific method is:
[0083] In a 10 mL microwave sealed tube, weigh 1 mmol of azido sugar 1b (2,3-O-isopropyl-5-azidofuran D-lyxose), dissolve it in 3 mL of anhydrous toluene, add triphenyl Base phosphine 393mg (1.5mmol), seal the tube at 80°C for 5-8min under microwave irradiation, add 146μL (2.0mmol) of thioglycolic acid, and continue the microwave seal reaction at 80°C for 10-15min. After the reaction was completed, anhydrous potassium carbonate powder was added to neutralize the reaction solution to pH=7, and dichloromethane (3×10 mL) was added for extraction, the organic phases were combined, and silica gel column chromatography (ethyl...
Embodiment 3
[0105] Synthesis of (5S,6R,7S,8S,8aR)-tetrahydro-5-trityloxymethyl-6-hydroxy-7,8-O-isopropyl-2H-thiazole[3,2-a] Pyridin-3(5)-one (compound 2c for short) and (5S,6R,7S,8S,8aS)-tetrahydro-5-trityloxymethyl-6-hydroxy-7,8-O-iso Propyl-2H-thiazo[3,2-a]pyridin-3(5H)-one (compound 3c for short)
[0106] Its chemical reaction process is as follows:
[0107]
[0108] The specific method is:
[0109] In a 10 mL microwave sealed tube, weigh 1 mmol azido sugar 1c (2,3-O-isopropyl-5-azido-6-O-tritylfuran D-mannose), and dissolve it in 3 mL Add 393 mg (1.5 mmol) of triphenylphosphine to anhydrous toluene, seal the tube at 80°C for microwave irradiation for 5-8 min., add 146 μL (2.0 mmol) of thioglycolic acid, and continue the microwave lock reaction at 80°C for 10-15 min. After the reaction was completed, anhydrous potassium carbonate powder was added to neutralize the reaction solution to pH=7, and dichloromethane (3×10 mL) was added for extraction, the organic phases were combined, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com