Method for extracting metallic lead from recovered waste lead-containing glass
A technology for extracting metal and glass, applied in the direction of improving process efficiency, can solve problems such as serious pollution, inability to remove lead, and lead pollution hazards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The used cathode ray tube (CRT) cone glass is used as raw material, and its chemical composition is shown in Table 1.
[0014] Table 1 Chemical composition of waste cathode ray tube (CRT) funnel glass
[0015]
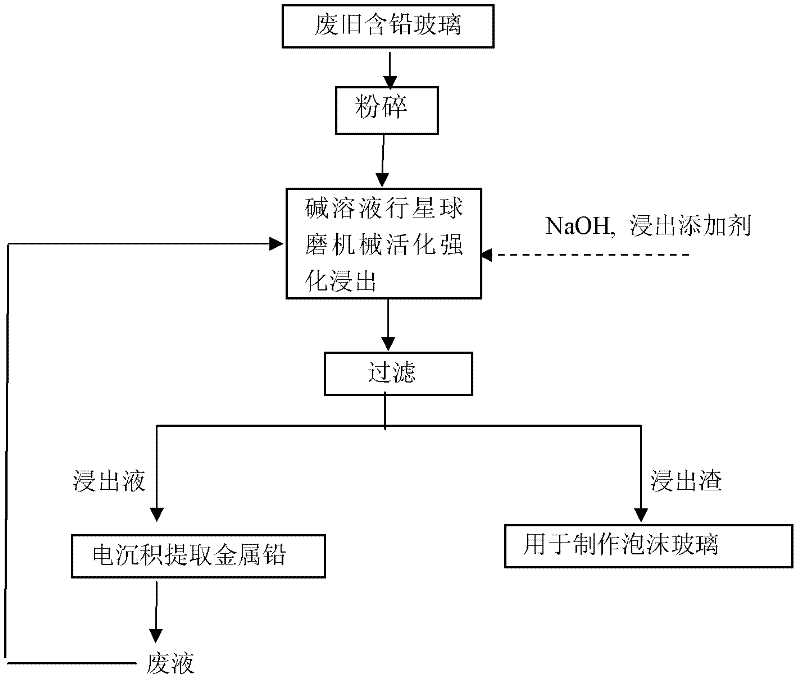
[0016] Extraction process such as figure 1 Shown: crush 50g of the above-mentioned glass raw material to 1-2mm, add it to the ball mill tank of the planetary ball mill, add 1.0L concentration of 3-7mol / L sodium hydroxide solution and 5g calcium-containing leaching reaction additive at the same time, the described The leaching reaction additive is commercially available industrial grade calcium oxide, wherein the mass ratio of raw material: ball milling medium is 1:8, and the ball milling medium is zirconia or stainless steel balls. Under the condition of planetary ball mill speed 150r / min, the reaction takes 120min. The leaching rate of lead in glass is 99.7%. Then filter, the leaching solution can be directly circulated in the next leaching process after t...
Embodiment 2
[0018] Using waste crystal glass as raw material, its chemical composition is shown in Table 2.
[0019] Table 2 Chemical composition of waste crystal glass
[0020]
[0021] Extraction process such as figure 1 Shown: crush 100g of the above-mentioned glass raw material to 1-2mm, add it to the ball mill tank of the planetary ball mill, add 1.8L of sodium hydroxide solution with a concentration of 3-7mol / L and 18g of calcium-containing leaching reaction additive at the same time, the The reaction additive is commercially available technical grade calcium hydroxide, and wherein raw material: the mass ratio of ball milling medium is 1: 10, and described ball milling medium is zirconia or stainless steel ball, and the planetary ball mill rotating speed is the stirring reaction under the condition of 250r / min 90min. The leaching rate of lead in glass is 99.9%. Then filter, the leaching solution can be directly circulated in the next leaching process after the metal lead is ex...
Embodiment 3
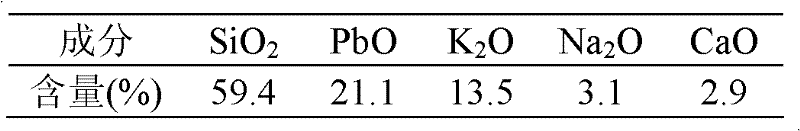
[0023] Using waste lead-containing glass as raw material, its chemical composition is shown in Table 3.
[0024] Table 3 Chemical composition of waste leaded glass
[0025]
[0026] Extraction process such as figure 1 Shown: crush 100g of the above-mentioned glass raw material to 1-2mm, add it to the ball mill tank of the planetary ball mill, add 0.8L of sodium hydroxide solution with a concentration of 3-7mol / L and 31g of calcium-containing leaching reaction additive at the same time, the The reaction additive is the mixture of commercially available technical grade calcium oxide and calcium hydroxide mixed in any proportion, wherein raw material: the mass ratio of ball milling medium is 1: 18, and described ball milling medium is zirconia or stainless steel ball, in planetary ball mill The reaction was stirred and reacted for 60 min at a rotation speed of 350 r / min. The leaching rate of lead in glass is 99.5%. Then filter, the leaching solution can be directly circulat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap