Two bromophenol compounds and application of pharmaceutically-acceptable salts of two bromophenol compounds in preparation of protection drug
A technology for reperfusion injury and myocardial ischemia, applied in the direction of drug combination, cardiovascular system diseases, ketone active ingredients, etc., can solve the problem of no relevant reports on the pharmacological effects of myocardial ischemia-reperfusion injury, and achieve the protection of myocardial ischemia Effects of reperfusion injury, inhibition of apoptosis and improvement of myocardial compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1. Effects of M48 and M49 on different physiological parameters of rats
[0016] 1.1 Experimental animals: 60 SD rats, half male and half male, were randomly divided into 10 groups, one of which was the sham operation group, and the other nine groups established SD rat myocardial ischemia-reperfusion injury models.
[0017] 1.2 Experimental grouping: one group of experimental drugs is the sham operation group, that is, the ligation is only passed through without ligation; the second group is the model group; the third group is the solvent control group; the fourth group is the M48 low-dose group 5mg / Kg; M48 medium dose group is 10mg / Kg; six groups are M48 high dose group 15mg / Kg; seven groups are M49 low dose group 5mg / Kg; eighth groups are M49 medium dose group 10mg / Kg; nine groups are M49 high dose group 15mg / Kg; Ten groups used the positive control drug sodium fructose diphosphate 10ml / Kg.
[0018] 1.3 Experimental method: Anesthetized by intraperitoneal injection ...
Embodiment 2
[0032] 1. Effects of M48 and M49 on myocardial infarction size in rats with myocardial ischemia-reperfusion
[0033] 1.1 Specific experiments:
[0034] The experimental animals and experimental groupings are the same as in 1 in Example 1, and the experimental method is basically the same as in 1 in Example 1, the difference is that in this experiment, 1% EvansBlue is intravenously injected after reperfusion of the corresponding group of experimental drugs , to stain the myocardium. Then counterstain with 1% TTC to analyze the infarct size, the calculation formula is as follows:
[0035] Infarct size = (infarct area / total myocardial area) × 100%
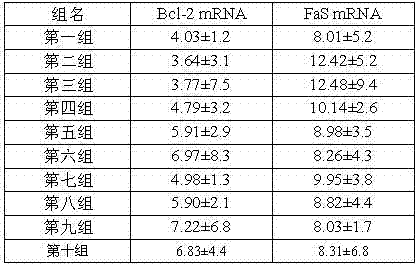
[0036] 1.2 Experimental results (Table 3):
[0037] Table 3: Effects of M48 and M49 on myocardial infarct size of rat myocardial ischemia-reperfusion
[0038]
[0039] As can be seen from the experimental results in Table 3: the four to ten groups of medication have significantly improved myocardial infarction area than the m...
Embodiment 3
[0047] 1. Effects of M48 and M49 on NO in myocardial tissue during myocardial ischemia-reperfusion in rats
[0048] 1.1 Specific experiments:
[0049] The experimental animals and the experimental groupings are all the same as the method of 1 in Example 1, and the experimental method is basically the same as the method of 1 in Example 1, except that the corresponding group of experimental drugs is reperfused to detect the myocardial tissue after 3 hours. Nitric Oxide (NO) indicator.
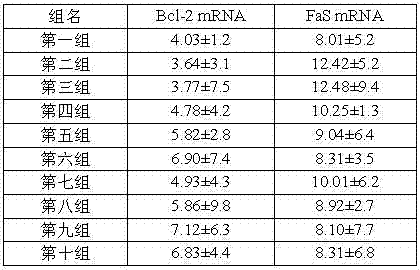
[0050] 1.2 Experimental results (Table 5):
[0051] Table 5: Effects of M48 and M49 on NO in myocardial tissue during myocardial ischemia-reperfusion in rats
[0052]
[0053] From the experimental results in Table 5, it can be seen that the area of myocardial infarction in groups 4 to 10 was significantly lower than that of the model control group (group 2), especially in the high-dose group, the level of NO was significantly reduced, indicating that M48 and M49 can inhibit myocardial inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com