N,N'-dipyrene butyryl lysine and application thereof
A dipyrene butyryl lysine, solution technology, applied in the field of the new compound nitrogen, nitrogen'-dipyrene butyryl lysine, can solve the detection interference of lead ions, fluorescence quenching, and the development of lead ion fluorescent probes Obstacles and other problems, to achieve the effect of being conducive to reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of Nitrogen, Nitrogen'-Dipyrenebutyryllysine
[0036]
[0037] The synthetic route of nitrogen, nitrogen '-dipyrene butyryl lysine is shown in formula (II), and concrete synthetic steps are as follows:
[0038] (1) Synthesis of Lysine Methyl Ester Hydrochloride
[0039]Add 5.0 mL of anhydrous methanol to a round bottom flask, cool in an ice-salt bath for 5 min, and slowly add 1.0 mL of thionyl chloride dropwise to it. After the dropwise addition, 1.0 g (6.85 mmol) of lysine solid was added to the above dichloromethane solution at one time. The mixed system was first stirred in an ice-salt bath for 10.0 min, and then stirred at room temperature for 2 h. Thereafter, the mixed solution was refluxed for 30 min under heating in a water bath at 70-80° C. and then cooled to room temperature. After most of the methanol in the solution was evaporated to dryness, 40.0 mL of anhydrous ether was added to the system, and a solid substance was precipitated. The mixed...
Embodiment 2
[0049] Detection method of lead ion concentration
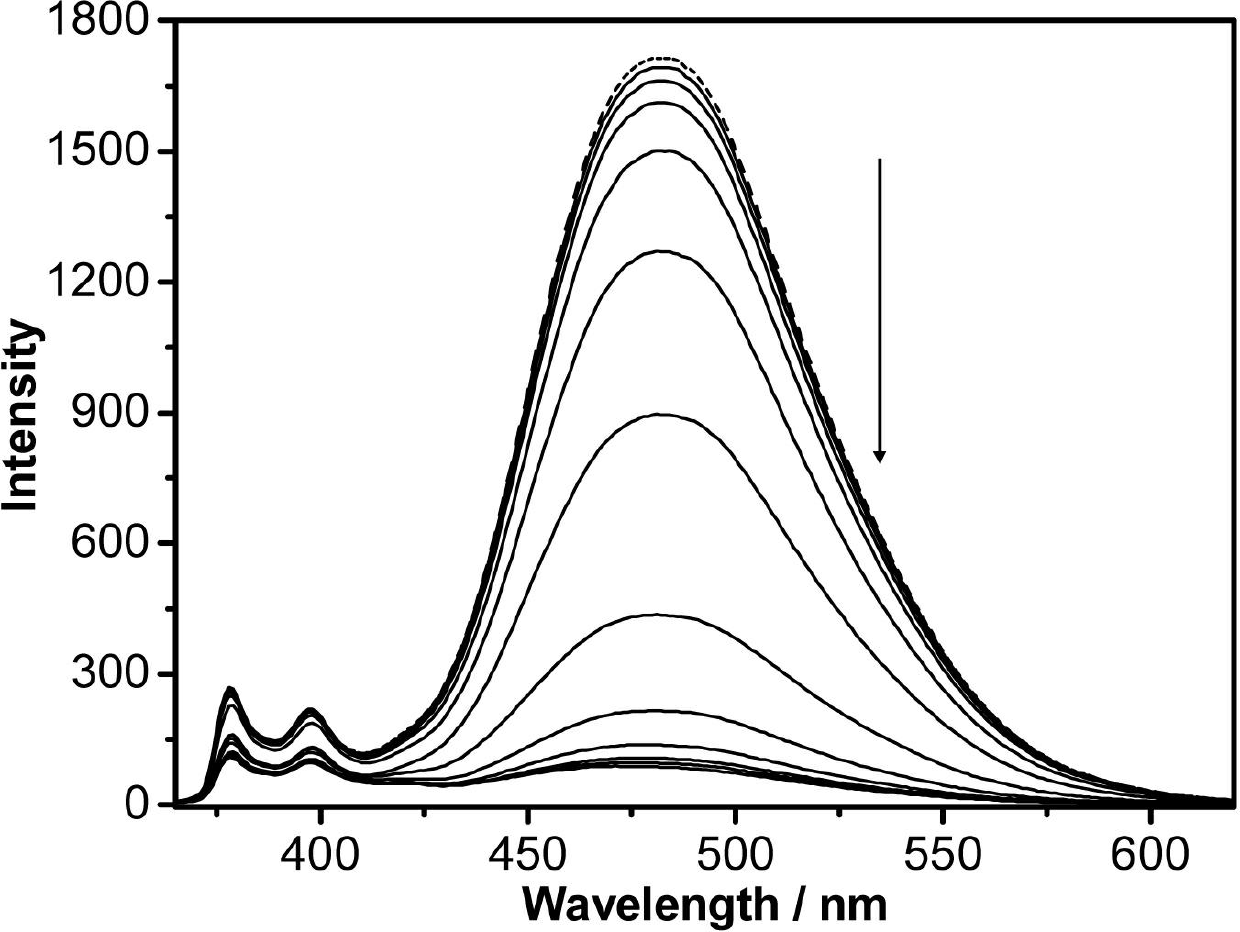
[0050] Titrate the concentrated solution of lead ions to a concentration of 2.0×10 -5 mol / L nitrogen, nitrogen'-dipyrenebutyryl lysine aqueous solution makes the concentration of lead ion respectively 0.0, 0.1, 0.25, 0.5, 1.0, 2.0, 3.0, 4.0, 8.0, 12.0, 16.0, 20.0, 40.0 μM (×10 -6 mol / L), and respectively detect their fluorescence intensity at the excitation wavelength of 350nm, and draw the fluorescence spectrum diagram, the fluorescence spectrum diagram obtained is as follows figure 1 shown.
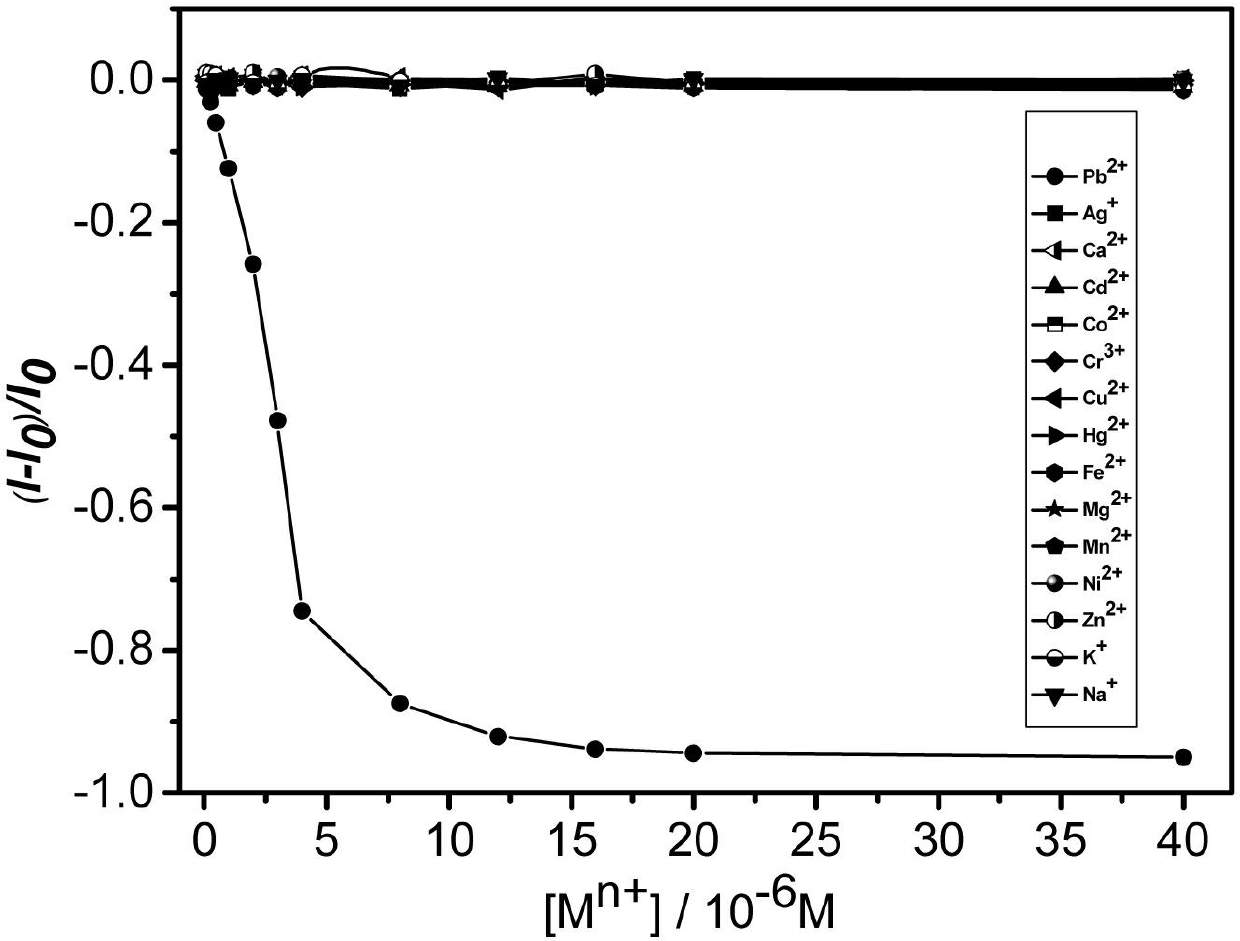
[0051] With the addition of lead ions, the fluorescence emission peak of nitrogen, nitrogen '-dipyrenebutyryl lysine weakens gradually and white flocculent precipitates (such as figure 2 shown). When the concentration ratio of the two is 1:1, the fluorescence can reach the maximum quenching, which is about 94.3%. From figure 1 It can be seen that nitrogen, nitrogen'-dipyrenebutyryl lysine showed high sensitivity to lead ions, an...
Embodiment 3
[0063] Lead Ion Precipitation Method Based on Nitrogen, Nitrogen'-Dipyrenebutyryllysine
[0064]Add nitrogen, nitrogen'-dipyrenebutyryl lysine solution into the solution containing lead ions, stir until no white flocculent precipitate is formed, which means the precipitation is complete, and centrifuge the suspension at 10000r / min for 5min , carrying out solid-liquid separation, the separated solid is the precipitation of nitrogen, nitrogen'-dipyrene butyryl lysine and lead ion complexes, and the obtained liquid is the solution from which lead ions have been removed. The results of ICP experiments show that nitrogen, nitrogen'-dipyrenebutyryl lysine has a removal rate of more than 94% for lead ions in solution. In the above-mentioned operation, the addition amount of nitrogen, nitrogen '-dipyrene butyryl lysine solution is so far that the lead ion precipitation in the solution can be made completely.
[0065] Of course, in addition to centrifugation, other methods such as fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com